STEM tomography reveals that the canalicular system and α-granules remain separate compartments during early secretion stages in blood platelets
- PMID: 26663480
- PMCID: PMC4829117
- DOI: 10.1111/jth.13225
STEM tomography reveals that the canalicular system and α-granules remain separate compartments during early secretion stages in blood platelets
Abstract
ESSENTIALS: How platelets organize their α-granule cargo and use their canalicular system remains controversial. Past structural studies were limited due to small sampling volumes or decreased resolution. Our analyses revealed homogeneous granules and a closed canalicular system that opened on activation. Understanding how platelets alter their membranes during activation and secretion elucidates hemostasis.
Background: Platelets survey the vasculature for damage and, in response, activate and release a wide range of proteins from their α-granules. Alpha-granules may be biochemically and structurally heterogeneous; however, other studies suggest that they may be more homogeneous with the observed variation reflecting granule dynamics rather than fundamental differences.
Objectives: Our aim was to address how the structural organization of α-granules supports their dynamics.
Methods: To preserve the native state, we prepared platelets by high-pressure freezing and freeze-substitution; and to image nearly entire cells, we recorded tomographic data in the scanning transmission electron microscope (STEM).
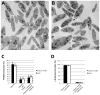
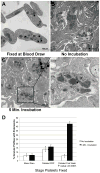
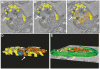
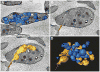
Results and conclusions: In resting platelets, we observed a morphologically homogeneous α-granule population that displayed little variation in overall matrix electron density in freeze-substituted preparations (i.e., macro-homogeneity). In resting platelets, the incidence of tubular granule extensions was low, ~4%, but this increased by > 10-fold during early steps in platelet secretion. Using STEM, we observed that the initially decondensing α-granules and the canalicular system remained as separate membrane domains. Decondensing α-granules were found to fuse heterotypically with the plasma membrane via long, tubular connections or homotypically with each other. The frequency of canalicular system fusion with the plasma membrane also increased by about three-fold. Our results validate the utility of freeze-substitution and STEM tomography for characterizing platelet granule secretion and suggest a model in which fusion of platelet α-granules with the plasma membrane occurs via long tubular connections that may provide a spatially limited access route for the timed release of α-granule proteins.
Keywords: blood platelets; cytoplasmic granules; electron microscope tomography; hemostasis; platelet activation.
© 2015 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
The authors declare no conflict of interest
Figures


References
-
- King S, Reed GL. Development of platelet secretory granules. Sem Cell Devel Biol. 2002;13:203–302. - PubMed
-
- Walsh PN, Gagnatelli G. Platelet antiheparin activity: storage site and release mechanism. Blood. 1974;44:157–168. - PubMed
-
- Kaplan KL, Broekman MJ, Chernoff A, Lesznik GR, Drillings M. Platelet alpha-granule proteins: studies on release and subcellular localization. Blood. 1979;53:604–618. - PubMed
-
- Heijnen HFG, Debili N, Vainchencker W, Breton-Gorius J, Geue HJ, Sixma JJ. Multivesicular bodies are an intermediate stage in the formation of platelet α-granules. Blood. 1988;91:2313–2328. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

