Macrophage and adipocyte IGF1 maintain adipose tissue homeostasis during metabolic stresses
- PMID: 26663512
- PMCID: PMC4793714
- DOI: 10.1002/oby.21354
Macrophage and adipocyte IGF1 maintain adipose tissue homeostasis during metabolic stresses
Abstract
Objective: Insulin-like growth factor-1 (IGF1) regulates differentiation and growth of tissues and reduces stress and injury. IGF1 also in a tissue-specific manner modulates the differentiation and lipid storage capacity of adipocytes in vitro, but its roles in adipose tissue development and response to stress are not known.
Methods: To study IGF1 in vivo, the cellular sources of adipose tissue Igf1 expression were identified and mice were generated with targeted deletion in adipocytes and macrophages. The effects of adipocyte and macrophage deficiency of IGF1 on adipose tissue development and the response to chronic (high-fat feeding) and acute (cold challenge) stress were studied.
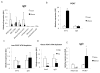
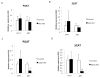
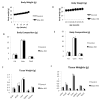
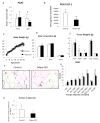
Results: The expression of Igf1 by adipose tissue was derived from multiple cell types including adipocytes and macrophages. In lean animals, adipocytes were the primary source of IGF1, but in obesity expression by adipocytes was reduced and by macrophages increased, so as to maintain overall adipose tissue Igf1 expression. Genetic deletion studies revealed that adipocyte-derived IGF1 regulated perigonadal but not subcutaneous adipose tissue mass during high-fat feeding and the development of obesity. Conversely, macrophage-derived IGF1 acutely modulated perigonadal adipose tissue mass during thermogenic challenges.
Conclusions: Local IGF1 is not required in lean adipose tissue development but is required to maintain homeostasis during both chronic and acute metabolic stresses.
© 2015 The Obesity Society.
Conflict of interest statement
Figures


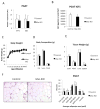
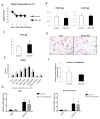
Comment in
-
IGF1 and adipose tissue homeostasis.Obesity (Silver Spring). 2016 Jan;24(1):10. doi: 10.1002/oby.21372. Epub 2015 Dec 5. Obesity (Silver Spring). 2016. PMID: 26638118 No abstract available.
References
-
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75(1):73–82. - PubMed
-
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75(1):59–72. - PubMed
-
- Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, et al. IGF-I is required for normal embryonic growth in mice. Genes & development. 1993;7(12B):2609–17. - PubMed
-
- Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(12):7088–92. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

