The mechanics of malaria parasite invasion of the human erythrocyte - towards a reassessment of the host cell contribution
- PMID: 26663815
- PMCID: PMC4819681
- DOI: 10.1111/cmi.12557
The mechanics of malaria parasite invasion of the human erythrocyte - towards a reassessment of the host cell contribution
Abstract
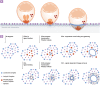
Despite decades of research, we still know little about the mechanics of Plasmodium host cell invasion. Fundamentally, while the essential or non-essential nature of different parasite proteins is becoming clearer, their actual function and how each comes together to govern invasion are poorly understood. Furthermore, in recent years an emerging world view is shifting focus away from the parasite actin-myosin motor being the sole force responsible for entry to an appreciation of host cell dynamics and forces and their contribution to the process. In this review, we discuss merozoite invasion of the erythrocyte, focusing on the complex set of pre-invasion events and how these might prime the red cell to facilitate invasion. While traditionally parasite interactions at this stage have been viewed simplistically as mediating adhesion only, recent work makes it apparent that by interacting with a number of host receptors and signalling pathways, combined with secretion of parasite-derived lipid material, that the merozoite may initiate cytoskeletal re-arrangements and biophysical changes in the erythrocyte that greatly reduce energy barriers for entry. Seen in this light Plasmodium invasion may well turn out to be a balance between host and parasite forces, much like that of other pathogen infection mechanisms.
© 2016 The Authors Cellular Microbiology Published by John Wiley & Sons Ltd.
Figures


References
-
- van den Akker, E. , Satchwell, T.J. , Williamson, R.C. , and Toye, A.M. (2010) Band 3 multiprotein complexes in the red cell membrane; of mice and men. Blood Cells Mol Dis 45: 1–8. - PubMed
-
- An, X. , Lecomte, M.C. , Chasis, J.A. , Mohandas, N. , and Gratzer, W. (2002) Shear‐response of the spectrin dimer‐tetramer equilibrium in the red blood cell membrane. J Biol Chem 277: 31796–31800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

