Assessment of Adverse Drug Reactions Based on Spontaneous Signals at Secondary Care Public Hospital
- PMID: 26664067
- PMCID: PMC4649787
- DOI: 10.4103/0250-474x.164771
Assessment of Adverse Drug Reactions Based on Spontaneous Signals at Secondary Care Public Hospital
Abstract
Adverse drug reactions are considered to be among the leading causes of morbidity and mortality. Approximately 5-25% of hospital admissions are due to adverse drug reactions and 6-15% of hospitalized patients experience serious adverse drug reactions, causing significant prolongation of hospital stay. Thus this study was aimed at determining adverse drug reactions by conducting spontaneous reporting in secondary care Govt. District Head Quarters Hospital at Ooty. A prospective Spontaneous Adverse Drug Reaction reporting study was conducted over a period of 12 months from July 2012 to June 2013. The assessment, categorization, causality, severity and preventability were assessed using standard criteria. A total of 47 suspected adverse drug reactions were reported during the study period. Over all incidences was 1.29% among the study population. Antibiotics (31.91%) were the class of drug most commonly involved, while ciprofloxacin (14.89%) was the most frequently reported. Type H (Hypersensitivity) reactions (51.06%) accounted for majority of the reports and a greater share of the adverse drug reactions are probable (89.36%) based on causality assessment. Mild reactions accounted 82.97% based on modified Hartwig and Siegel severity scale. In 76.59% of the reports, the reaction was considered to be preventable based on Schumock and Thornton preventability scale. The implementation of monitoring based on spontaneous reporting will be useful for the detection and evaluation is associated with increase in morbidity and duration of hospitalization. This study also has established the vital role of clinical pharmacist in the adverse drug reaction monitoring program.
Keywords: Adverse drug reactions; Naranjo's scale; Wills and Brown classification; inpatient; secondary care hospital; spontaneous reporting.
Figures
References
-
- American College of Clinical Pharmacy. The definition of clinical pharmacy. Pharmacotherapy. 2008;28:816–7. - PubMed
-
- Parthasarathi G, Nyfort-Hansen K, Nahata M, editors. Hyderabad: Orient Blackswan; 2008. A Text Book of Clinical Pharmacy Practice-essential Concept and Skills; p. 09.
-
- Beijer HJ, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): A meta-analysis of observational studies. Pharm World Sci. 2002;24:46–54. - PubMed
-
- Edwards IR, Aronson JK. Adverse drug reactions: Definitions, diagnosis, and management. Lancet. 2000;356:1255–9. - PubMed
-
- van Grootheest K, Olsson S, Couper M, de Jong-van den Berg L. Pharmacists’ role in reporting adverse drug reactions in an international perspective. Pharmacoepidemiol Drug Saf. 2004;13:457–64. - PubMed
LinkOut - more resources
Full Text Sources

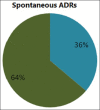
 male percentage
male percentage  female percentage.
female percentage.