Pictorial essay of radiological features of benign intrathoracic masses
- PMID: 26664560
- PMCID: PMC4652288
- DOI: 10.4103/1817-1737.160365
Pictorial essay of radiological features of benign intrathoracic masses
Abstract
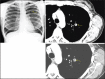
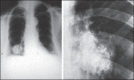
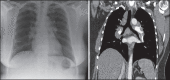
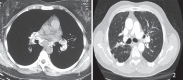
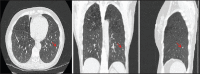
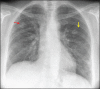
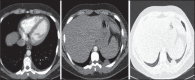
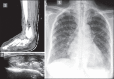
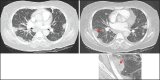
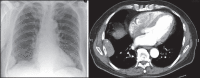
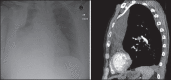
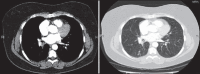
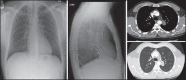
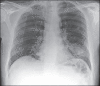
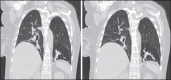
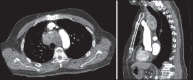
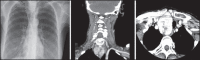
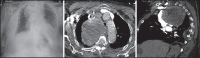
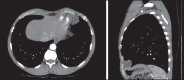
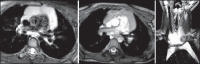
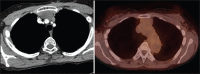
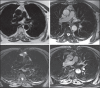
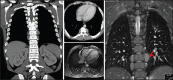
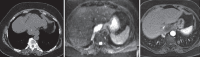
With increased exposure of patients to routine imaging, incidental benign intrathoracic masses are frequently recognized. Most have classical imaging features, which are pathognomonic for their benignity. The aim of this pictorial review is to educate the reader of radiological features of several types of intrathoracic masses. The masses are categorized based on their location/origin and are grouped into parenchymal, pleural, mediastinal, or bronchial. Thoracic wall masses that invade the thorax such as neurofibromas and lipomas are included as they may mimic intrathoracic masses. All examples are illustrated and include pulmonary hamartoma, pleural fibroma, sarcoidosis, bronchial carcinoid, and bronchoceles together with a variety of mediastinal cysts on plain radiographs, computed tomography (CT) and magnetic resonance imaging (MRI). Sometimes a multimodality approach would be needed to confirm the diagnosis in atypical cases. The study would include the incorporation of radionuclide studies and relevant discussion in a multidisciplinary setting.
Keywords: Intrathoracic masses; pulmonary mass; radiology.
Conflict of interest statement
Figures


















References
-
- Leader JK, Warfel TE, Fuhrman CR, Golla SK, Weissfeld JL, Avila RS, et al. Pulmonary nodule detection with low-dose CT of the lung: Agreement among radiologists. AJR Am J Roentgenol. 2005;185:973–8. - PubMed
-
- Salahudeen HM, Hoey ET, Robertson RJ, Darby MJ. CT appearances of pleural tumours. Clin Radiol. 2009;64:918–30. - PubMed
-
- Helm EJ, Matin TN, Gleeson FV. Imaging of the pleura. J Magn Reson Imaging. 2010;32:1275–86. - PubMed
-
- Maffione AM, Grassetto G, Rampin L, Chondrogiannis S, Marzola MC, Ambrosini V, et al. Molecular imaging of pulmonary nodules. AJR Am J Roentgenol. 2014;202:W217–23. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

