Preparative semiconductor photoredox catalysis: An emerging theme in organic synthesis
- PMID: 26664577
- PMCID: PMC4660884
- DOI: 10.3762/bjoc.11.173
Preparative semiconductor photoredox catalysis: An emerging theme in organic synthesis
Abstract
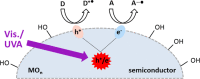
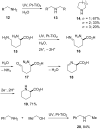
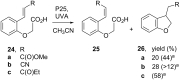
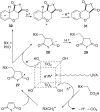
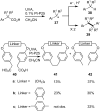
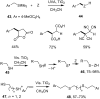
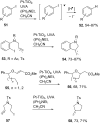
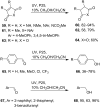
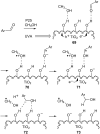
Heterogeneous semiconductor photoredox catalysis (SCPC), particularly with TiO2, is evolving to provide radically new synthetic applications. In this review we describe how photoactivated SCPCs can either (i) interact with a precursor that donates an electron to the semiconductor thus generating a radical cation; or (ii) interact with an acceptor precursor that picks up an electron with production of a radical anion. The radical cations of appropriate donors convert to neutral radicals usually by loss of a proton. The most efficient donors for synthetic purposes contain adjacent functional groups such that the neutral radicals are resonance stabilized. Thus, ET from allylic alkenes and enol ethers generated allyl type radicals that reacted with 1,2-diazine or imine co-reactants to yield functionalized hydrazones or benzylanilines. SCPC with tertiary amines enabled electron-deficient alkenes to be alkylated and furoquinolinones to be accessed. Primary amines on their own led to self-reactions involving C-N coupling and, with terminal diamines, cyclic amines were produced. Carboxylic acids were particularly fruitful affording C-centered radicals that alkylated alkenes and took part in tandem addition cyclizations producing chromenopyrroles; decarboxylative homo-dimerizations were also observed. Acceptors initially yielding radical anions included nitroaromatics and aromatic iodides. The latter led to hydrodehalogenations and cyclizations with suitable precursors. Reductive SCPC also enabled electron-deficient alkenes and aromatic aldehydes to be hydrogenated without the need for hydrogen gas.
Keywords: carboxylic acids; free radicals; organic synthesis; photocatalysis; titania.
Figures


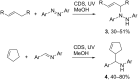



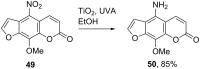
Similar articles
-
Semiconductor Photocatalysis for Chemoselective Radical Coupling Reactions.Acc Chem Res. 2017 Apr 18;50(4):1002-1010. doi: 10.1021/acs.accounts.7b00023. Epub 2017 Apr 5. Acc Chem Res. 2017. PMID: 28378591
-
When Light Meets Nitrogen-Centered Radicals: From Reagents to Catalysts.Acc Chem Res. 2020 May 19;53(5):1066-1083. doi: 10.1021/acs.accounts.0c00090. Epub 2020 Apr 14. Acc Chem Res. 2020. PMID: 32286794
-
Synthetic Utilization of α-Aminoalkyl Radicals and Related Species in Visible Light Photoredox Catalysis.Acc Chem Res. 2016 Sep 20;49(9):1946-56. doi: 10.1021/acs.accounts.6b00251. Epub 2016 Aug 9. Acc Chem Res. 2016. PMID: 27505299
-
The chemistry of amine radical cations produced by visible light photoredox catalysis.Beilstein J Org Chem. 2013 Oct 1;9:1977-2001. doi: 10.3762/bjoc.9.234. Beilstein J Org Chem. 2013. PMID: 24204409 Free PMC article. Review.
-
Photoredox-Mediated Routes to Radicals: The Value of Catalytic Radical Generation in Synthetic Methods Development.ACS Catal. 2017 Apr 7;7(4):2563-2575. doi: 10.1021/acscatal.7b00094. Epub 2017 Mar 14. ACS Catal. 2017. PMID: 28413692 Free PMC article. Review.
Cited by
-
Synthetic Strategies for 5- and 6-Membered Ring Azaheterocycles Facilitated by Iminyl Radicals.Molecules. 2016 May 18;21(5):660. doi: 10.3390/molecules21050660. Molecules. 2016. PMID: 27213311 Free PMC article. Review.
-
Visible-Light-Mediated [4+2] Annulation of N-Cyclobutylanilines with Alkynes Catalyzed by Self-Doped Ti3+ @TiO2.Chemistry. 2017 Nov 2;23(61):15396-15403. doi: 10.1002/chem.201701587. Epub 2017 Aug 9. Chemistry. 2017. PMID: 28608493 Free PMC article.
-
Linker-Assisted CdS-TiO2 Nanohybrids as Reusable Visible Light Photocatalysts for the Oxidative Hydroxylation of Arylboronic Acids.J Org Chem. 2023 May 19;88(10):6489-6497. doi: 10.1021/acs.joc.2c02964. Epub 2023 Mar 17. J Org Chem. 2023. PMID: 36930860 Free PMC article.
-
Functionalised Oximes: Emergent Precursors for Carbon-, Nitrogen- and Oxygen-Centred Radicals.Molecules. 2016 Jan 7;21(1):63. doi: 10.3390/molecules21010063. Molecules. 2016. PMID: 26751437 Free PMC article. Review.
-
Synthetic Approaches for C-N Bonds by TiO2 Photocatalysis.Front Chem. 2019 Sep 18;7:635. doi: 10.3389/fchem.2019.00635. eCollection 2019. Front Chem. 2019. PMID: 31620428 Free PMC article. Review.
References
-
- Nicewicz D A, Nguyen T M. ACS Catal. 2014;4:355–360. doi: 10.1021/cs400956a. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
