Neural Coding of Perceived Odor Intensity
- PMID: 26665162
- PMCID: PMC4672005
- DOI: 10.1523/ENEURO.0083-15.2015
Neural Coding of Perceived Odor Intensity
Abstract
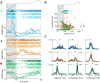
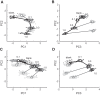
Stimulus intensity is a fundamental perceptual feature in all sensory systems. In olfaction, perceived odor intensity depends on at least two variables: odor concentration; and duration of the odor exposure or adaptation. To examine how neural activity at early stages of the olfactory system represents features relevant to intensity perception, we studied the responses of mitral/tufted cells (MTCs) while manipulating odor concentration and exposure duration. Temporal profiles of MTC responses to odors changed both as a function of concentration and with adaptation. However, despite the complexity of these responses, adaptation and concentration dependencies behaved similarly. These similarities were visualized by principal component analysis of average population responses and were quantified by discriminant analysis in a trial-by-trial manner. The qualitative functional dependencies of neuronal responses paralleled psychophysics results in humans. We suggest that temporal patterns of MTC responses in the olfactory bulb contribute to an internal perceptual variable: odor intensity.
Keywords: Concentration versus adaptation; extracellular electrophysiology; human psychophysics; olfactory bulb.
Figures







References
-
- Beck A, Kruger L, Calabresi P (1954) Observations on olfactory intensity. I. Training procedure, methods, and data for two aliphatic homologous series. Ann N Y Acad Sci 58:225–238. - PubMed
-
- Bodyak N, Slotnick B (1999) Performance of mice in an automated olfactometer: odor detection, discrimination and odor memory. Chem Senses 24:637–645. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
