Molecular and genomic characterization of pathogenic traits of group A Streptococcus pyogenes
- PMID: 26666305
- PMCID: PMC4773581
- DOI: 10.2183/pjab.91.539
Molecular and genomic characterization of pathogenic traits of group A Streptococcus pyogenes
Abstract
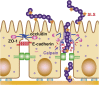
Group A streptococcus (GAS) or Streptococcus pyogenes causes various diseases ranging from self-limiting sore throat to deadly invasive diseases. The genome size of GAS is 1.85-1.9 Mb, and genomic rearrangement has been demonstrated. GAS possesses various surface-associated substances such as hyaluronic capsule, M proteins, and fibronectin/laminin/immunoglobulin-binding proteins. These are related to the virulence and play multifaceted and mutually reflected roles in the pathogenesis of GAS infections. Invasion of GAS into epithelial cells and deeper tissues provokes immune and non-immune defense or inflammatory responses including the recruitment of neutrophils, macrophages, and dendritic cells in hosts. GAS frequently evades host defense mechanisms by using its virulence factors. Extracellular products of GAS may perturb cellular and subcellular functions and degrade tissues enzymatically, which leads to the aggravation of local and/or systemic disorders in the host. In this review, we summarize some important cellular and extracellular substances that may affect pathogenic processes during GAS infections, and the host responses to these.
Figures



References
-
- Kawamura Y., Hou X.-G., Sultana F., Miura H., Ezaki T. (1995) Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int. J. Syst. Bacteriol. 45, 406–408. - PubMed
-
- Olafsdottir L.B., Erlendsdóttir H., Melo-Cristino J., Weinberger D.M., Ramirez M., Kristinsson K.G., Gottfredsson M. (2014) Invasive infections due to Streptococcus pyogenes: seasonal variation of severity and clinical characteristics, Iceland, 1975 to 2012. Euro Surveill. 19, pii=20784. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

