Structure-function correlations of rat trigeminal primary neurons: Emphasis on club-like endings, a vibrissal mechanoreceptor
- PMID: 26666306
- PMCID: PMC4773582
- DOI: 10.2183/pjab.91.560
Structure-function correlations of rat trigeminal primary neurons: Emphasis on club-like endings, a vibrissal mechanoreceptor
Erratum in
-
Errata to "Structure-function correlations of rat trigeminal primary neurons: Emphasis on club-like endings, a vibrissal mechanoreceptor".Proc Jpn Acad Ser B Phys Biol Sci. 2016;92(2):76. doi: 10.2183/pjab.92.76. Proc Jpn Acad Ser B Phys Biol Sci. 2016. PMID: 26860456 Free PMC article. No abstract available.
Abstract
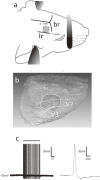
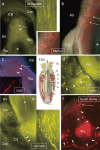
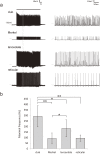
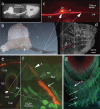
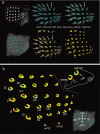
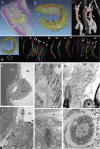
This study focuses on the structure and function of the primary sensory neurons that innervate vibrissal follicles in the rat. Both the peripheral and central terminations, as well as their firing properties were identified using intracellular labelling and recording in trigeminal ganglia in vivo. Fifty-one labelled neurons terminating peripherally, as club-like, Merkel, lanceolate, reticular or spiny endings were identified by their morphology. All neurons responded robustly to air puff stimulation applied to the vibrissal skin. Neurons with club-like endings responded with the highest firing rates; their peripheral processes rarely branched between the cell body and their terminal tips. The central branches of these neurons displayed abundant collaterals terminating within all trigeminal nuclei. Analyses of three-dimensional reconstructions reveal a palisade arrangement of club-like endings bound to the ringwulst by collagen fibers. Our morphological findings suggest that neurons with club-like endings sense mechanical aspects related to the movement of the ringwulst and convey this information to all trigeminal nuclei in the brainstem.
Figures

Similar articles
-
The Cellular and Mechanical Basis for Response Characteristics of Identified Primary Afferents in the Rat Vibrissal System.Curr Biol. 2020 Mar 9;30(5):815-826.e5. doi: 10.1016/j.cub.2019.12.068. Epub 2020 Jan 30. Curr Biol. 2020. PMID: 32004452 Free PMC article.
-
Innervation of the maxillary vibrissae in mice as revealed by anterograde and retrograde tract tracing.Cell Tissue Res. 2004 Feb;315(2):167-80. doi: 10.1007/s00441-003-0816-z. Epub 2003 Nov 11. Cell Tissue Res. 2004. PMID: 14610665
-
Innervation of a single vibrissa in the whisker-pad of rats.Neuroreport. 2000 Mar 20;11(4):849-51. doi: 10.1097/00001756-200003200-00038. Neuroreport. 2000. PMID: 10757532
-
Calcium signaling in terminal Schwann cells associated with lanceolate sensory endings in rat vibrissae.Ital J Anat Embryol. 2005;110(2 Suppl 1):19-24. Ital J Anat Embryol. 2005. PMID: 16101016
-
Effect of neonatal capsaicin and infraorbital nerve section on whisker-related patterns in the rat trigeminal nucleus.J Comp Neurol. 1997 Sep 8;385(4):599-615. doi: 10.1002/(sici)1096-9861(19970908)385:4<599::aid-cne6>3.0.co;2-z. J Comp Neurol. 1997. PMID: 9302107 Review.
Cited by
-
Active Touch and Self-Motion Encoding by Merkel Cell-Associated Afferents.Neuron. 2017 May 3;94(3):666-676.e9. doi: 10.1016/j.neuron.2017.03.045. Epub 2017 Apr 20. Neuron. 2017. PMID: 28434802 Free PMC article.
-
Distribution, fine structure, and three-dimensional innervation of lamellar corpuscles in rat plantar skin.Cell Tissue Res. 2021 Dec;386(3):477-490. doi: 10.1007/s00441-021-03525-5. Epub 2021 Sep 25. Cell Tissue Res. 2021. PMID: 34562148
-
Supra-orbital whiskers act as wind-sensing antennae in rats.PLoS Biol. 2023 Jul 6;21(7):e3002168. doi: 10.1371/journal.pbio.3002168. eCollection 2023 Jul. PLoS Biol. 2023. PMID: 37410722 Free PMC article.
-
Angular Tuning Properties of Low Threshold Mechanoreceptors in Isolated Rat Whisker Hair Follicles.eNeuro. 2022 Nov 28;9(6):ENEURO.0175-22.2022. doi: 10.1523/ENEURO.0175-22.2022. Print 2022 Nov-Dec. eNeuro. 2022. PMID: 36376066 Free PMC article.
-
Interactions of Whisking and Touch Signals in the Rat Brainstem.J Neurosci. 2021 Jun 2;41(22):4826-4839. doi: 10.1523/JNEUROSCI.1410-20.2021. Epub 2021 Apr 23. J Neurosci. 2021. PMID: 33893218 Free PMC article.
References
-
- Retzius, G. (1880) Untersuchungen uber die- Norvensellen der cerebrospinalen. Ganglien und der ubrigen periferschen. Kopfganglien mit besonderer. Berucksichtigung auf die zellenaus laufer. In Arch. Anat. Physiol., Antomiche Abtheilung (eds. His, W., Braune, W. and Bois-Reymond, E.D.). Verlag von Veit & Comp., Leipzig, pp. 369–402.
-
- Rice F.L., Munger B.L. (1986) A comparative light microscopic analysis of the sensory innervation of the mystacial pad. I. Innervation of vibrissal follicle-sinus complexes. J. Comp. Neurol. 252, 186–205. - PubMed
-
- Rice F.L., Kinnman E., Aldskogius H., Johansson O., Arvidsson J. (1993) The innervation of the mystacial pad of the rat as revealed by PGP 9.5 immunofluorescence. J. Comp. Neurol. 337, 366–385. - PubMed
-
- Fundin B.T., Arvidsson J., Rice F.L. (1995) Innervation of nonmystacial vibrissae in the adult rat. J. Comp. Neurol. 357, 501–512. - PubMed
-
- Rice F.L., Fundin B.T., Arvidsson J., Aldskogius H., Johansson O. (1997) Comprehensive immunofluorescence and lectin binding analysis of vibrissal follicle sinus complex innervation in the mystacial pad of the rat. J. Comp. Neurol. 385, 149–184. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

