The LIPPSMAck POP (Lung Infection Prevention Post Surgery - Major Abdominal - with Pre-Operative Physiotherapy) trial: study protocol for a multi-centre randomised controlled trial
- PMID: 26666321
- PMCID: PMC4678689
- DOI: 10.1186/s13063-015-1090-6
The LIPPSMAck POP (Lung Infection Prevention Post Surgery - Major Abdominal - with Pre-Operative Physiotherapy) trial: study protocol for a multi-centre randomised controlled trial
Abstract
Background: Post-operative pulmonary complications are a significant problem following open upper abdominal surgery. Preliminary evidence suggests that a single pre-operative physiotherapy education and preparatory lung expansion training session alone may prevent respiratory complications more effectively than supervised post-operative breathing and coughing exercises. However, the evidence is inconclusive due to methodological limitations. No well-designed, adequately powered, randomised controlled trial has investigated the effect of pre-operative education and training on post-operative respiratory complications, hospital length of stay, and health-related quality of life following upper abdominal surgery.
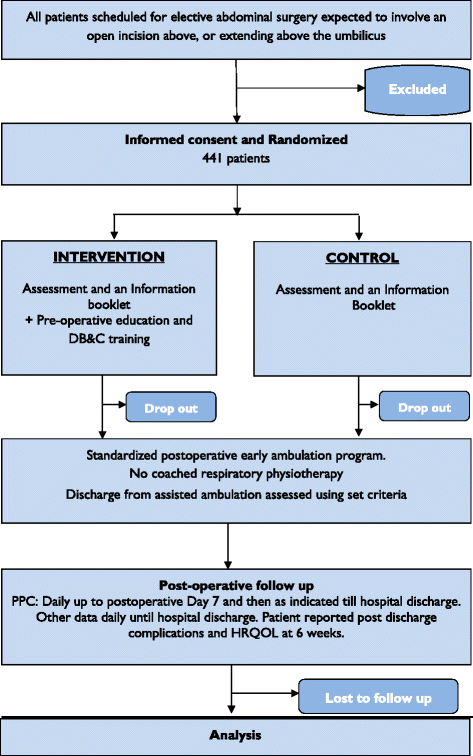
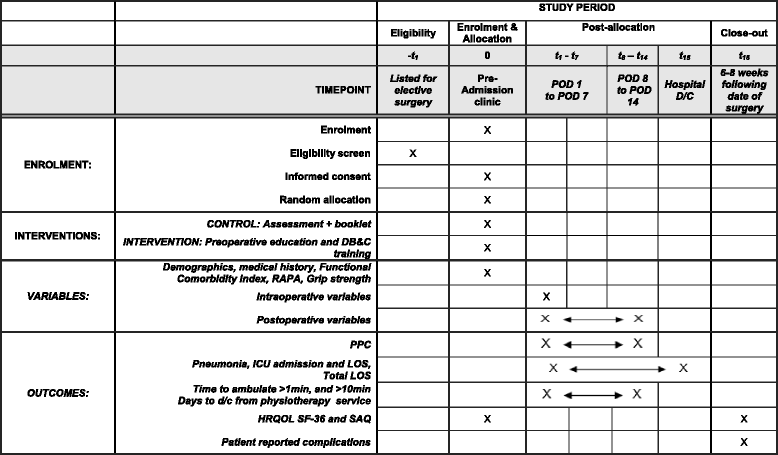
Methods/design: The Lung Infection Prevention Post Surgery - Major Abdominal- with Pre-Operative Physiotherapy (LIPPSMAck POP) trial is a pragmatic, investigator-initiated, bi-national, multi-centre, patient- and assessor-blinded, parallel group, randomised controlled trial, powered for superiority. Four hundred and forty-one patients scheduled for elective open upper abdominal surgery at two Australian and one New Zealand hospital will be randomised using concealed allocation to receive either i) an information booklet or ii) an information booklet, plus one additional pre-operative physiotherapy education and training session. The primary outcome is respiratory complication incidence using standardised diagnostic criteria. Secondary outcomes include hospital length of stay and costs, pneumonia diagnosis, intensive care unit readmission and length of stay, days/h to mobilise >1 min and >10 min, and, at 6 weeks post-surgery, patient reported complications, health-related quality of life, and physical capacity.
Discussion: The LIPPSMAck POP trial is a multi-centre randomised controlled trial powered and designed to investigate whether a single pre-operative physiotherapy session prevents post-operative respiratory complications. This trial standardises post-operative assisted ambulation and physiotherapy, measures many known confounders, and includes a post-discharge follow-up of complication rates, functional capacity, and health-related quality of life. This trial is currently recruiting.
Trial registration: Australian New Zealand Clinical Trials Registry number: ACTRN12613000664741 , 19 June 2013.
Figures
References
-
- AIHW. Procedures Cubes 2011–2012: National Hospital Morbidity Database. 2013. http://www.aihw.gov.au/hospitals-data/procedures-data-cubes/#ardrglink. Accessed 7 September 2014.
-
- Scholes RL, Browning L, Sztendur EM, Denehy L. Duration of anaesthesia, type of surgery, respiratory co-morbidity, predicted VO max and smoking predict postoperative pulmonary complications after upper abdominal surgery: an observational study. Aust J Physiother. 2009;55(3):191–8. doi: 10.1016/S0004-9514(09)70081-9. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

