Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy
- PMID: 26666572
- PMCID: PMC4682118
- DOI: 10.1038/ncomms10145
Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy
Abstract
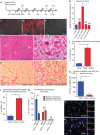
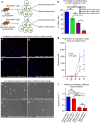
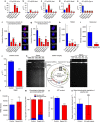
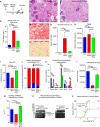
Sepsis, or systemic inflammatory response syndrome, is the major cause of critical illness resulting in admission to intensive care units. Sepsis is caused by severe infection and is associated with mortality in 60% of cases. Morbidity due to sepsis is complicated by neuromyopathy, and patients face long-term disability due to muscle weakness, energetic dysfunction, proteolysis and muscle wasting. These processes are triggered by pro-inflammatory cytokines and metabolic imbalances and are aggravated by malnutrition and drugs. Skeletal muscle regeneration depends on stem (satellite) cells. Herein we show that mitochondrial and metabolic alterations underlie the sepsis-induced long-term impairment of satellite cells and lead to inefficient muscle regeneration. Engrafting mesenchymal stem cells improves the septic status by decreasing cytokine levels, restoring mitochondrial and metabolic function in satellite cells, and improving muscle strength. These findings indicate that sepsis affects quiescent muscle stem cells and that mesenchymal stem cells might act as a preventive therapeutic approach for sepsis-related morbidity.
Figures
References
-
- Cohen J. The immunopathogenesis of sepsis. Nature 420, 885–891 (2002). - PubMed
-
- Gamrin L. et al. Longitudinal changes of biochemical parameters in muscle during critical illness. Metabolism 46, 756–762 (1997). - PubMed
-
- Mittendorfer B., Gore D. C., Herndon D. N. & Wolfe R. R. Accelerated glutamine synthesis in critically ill patients cannot maintain normal intramuscular free glutamine concentration. JPEN J. Parenter. Enteral Nutr. 23, 243–250 discussion 250–242 (1999). - PubMed
-
- Rennie M. J. et al. Glutamine metabolism and transport in skeletal muscle and heart and their clinical relevance. J. Nutr. 126, 1142S–1149S (1996). - PubMed
-
- Windmueller H. G. & Spaeth A. E. Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. Quantitative importance of glutamine, glutamate, and aspartate. J. Biol. Chem. 255, 107–112 (1980). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

