The significance of G-CSF expression and myeloid-derived suppressor cells in the chemoresistance of uterine cervical cancer
- PMID: 26666576
- PMCID: PMC4678366
- DOI: 10.1038/srep18217
The significance of G-CSF expression and myeloid-derived suppressor cells in the chemoresistance of uterine cervical cancer
Abstract
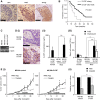
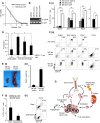
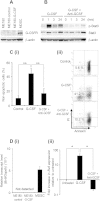
Granulocyte-colony stimulating factor (G-CSF) producing malignant tumor has been reported to occur in various organs, and has been associated with poor clinical outcome. The aim of this study is to investigate the significance of tumor G-CSF expression in the chemosensitivity of uterine cervical cancer. The clinical data of recurrent or advanced cervical cancer patients who were treated with platinum-based chemotherapy were analyzed. Clinical samples, cervical cancer cell lines, and a mouse model of cervical cancer were employed to examine the mechanisms responsible for the development of chemoresistance in G-CSF-producing cervical cancer, focusing on myeloid-derived suppressor cells (MDSC). As a result, the tumor G-CSF expression was significantly associated with increased MDSC frequencies and compromised survival. In vitro and in vivo experiments demonstrated that the increased MDSC induced by tumor-derived G-CSF is involved in the development of chemoresistance. The depletion of MDSC via splenectomy or the administration of anti-Gr-1 antibody sensitized G-CSF-producing cervical cancer to cisplatin. In conclusion, tumor G-CSF expression is an indicator of an extremely poor prognosis in cervical cancer patients that are treated with chemotherapy. Combining MDSC-targeting treatments with current standard chemotherapies might have therapeutic efficacy as a treatment for G-CSF-producing cervical cancer.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Monk B. J., Huang H. Q., Cella D. & Long H. J. & Study, G. O. G. Quality of life outcomes from a randomized phase III trial of cisplatin with or without topotecan in advanced carcinoma of the cervix: a Gynecologic Oncology Group Study. J Clin Oncol 23, 4617–4625, 10.1200/JCO.2005.10.522 (2005). - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

