Carbon regulation of environmental pH by secreted small molecules that modulate pathogenicity in phytopathogenic fungi
- PMID: 26666972
- PMCID: PMC6638356
- DOI: 10.1111/mpp.12355
Carbon regulation of environmental pH by secreted small molecules that modulate pathogenicity in phytopathogenic fungi
Abstract
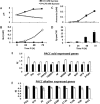
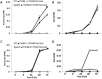
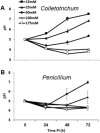
Fruit pathogens can contribute to the acidification or alkalinization of the host environment. This capability has been used to divide fungal pathogens into acidifying and/or alkalinizing classes. Here, we show that diverse classes of fungal pathogens-Colletotrichum gloeosporioides, Penicillium expansum, Aspergillus nidulans and Fusarium oxysporum-secrete small pH-affecting molecules. These molecules modify the environmental pH, which dictates acidic or alkaline colonizing strategies, and induce the expression of PACC-dependent genes. We show that, in many organisms, acidification is induced under carbon excess, i.e. 175 mm sucrose (the most abundant sugar in fruits). In contrast, alkalinization occurs under conditions of carbon deprivation, i.e. less than 15 mm sucrose. The carbon source is metabolized by glucose oxidase (gox2) to gluconic acid, contributing to medium acidification, whereas catalysed deamination of non-preferred carbon sources, such as the amino acid glutamate, by glutamate dehydrogenase 2 (gdh2), results in the secretion of ammonia. Functional analyses of Δgdh2 mutants showed reduced alkalinization and pathogenicity during growth under carbon deprivation, but not in high-carbon medium or on fruit rich in sugar, whereas analysis of Δgox2 mutants showed reduced acidification and pathogencity under conditions of excess carbon. The induction pattern of gdh2 was negatively correlated with the expression of the zinc finger global carbon catabolite repressor creA. The present results indicate that differential pH modulation by fruit fungal pathogens is a host-dependent mechanism, affected by host sugar content, that modulates environmental pH to enhance fruit colonization.
Keywords: carbon regulation of pathogenicity; pH regulation; pathogenicity.
© 2015 BSPP and John Wiley & Sons Ltd.
Figures






References
-
- Alkan, N. , Fluhr, R. , Sherman, A. and Prusky, D. (2008) Role of ammonia secretion and pH modulation on pathogenicity of Colletotrichum coccodes on tomato fruit. Mol. Plant–Microbe Interact. 21, 1058–1066. - PubMed
-
- Alkan, N. , Davydov, O. , Sagi, M. , Fluhr, R. and Prusky, D. (2009) Ammonium secretion by Colletotrichum coccodes activates host NADPH oxidase activity enhancing host cell death and fungal virulence in tomato fruits Mol. Plant–Microbe Interact. 12, 1484–1491. - PubMed
-
- Alkan, N. , Espeso, E.A. and Prusky, D. (2013a) Virulence regulation of phytopathogenic fungi by pH. Antioxid. Redox Signal. 19, 1012–1025. - PubMed
-
- Alkan, N. , Meng, X. , Friedlander, G. , Reuveni, E. , Sukno, S. , Sherman, A ., Thon, M., Fluhr, R. and Prusky, D. (2013b) Global aspects of pacC regulation of pathogenicity genes in Colletotrichum gloeosporioides as revealed by transcriptome analysis. Mol. Plant–Microbe Interact. 26, 1345–1358. - PubMed
-
- Alkan, N. , Friedlander, G. , Ment, D. , Prusky, D. and Fluhr, R. (2015) Simultaneous transcriptome analysis of Colletotrichum gloeosporioides and tomato fruit pathosystem reveals novel fungal pathogenicity and fruit defense strategies. New Phytol. 205, 801–815. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

