Cannabis and cocaine decrease cognitive impulse control and functional corticostriatal connectivity in drug users with low activity DBH genotypes
- PMID: 26667034
- PMCID: PMC5167221
- DOI: 10.1007/s11682-015-9488-z
Cannabis and cocaine decrease cognitive impulse control and functional corticostriatal connectivity in drug users with low activity DBH genotypes
Abstract
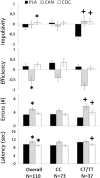
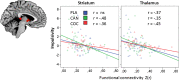
The dopamine β-hydroxylase (DβH) enzyme transforms dopamine into noradrenaline. We hypothesized that individuals with low activity DBH genotypes (rs1611115 CT/TT) are more sensitive to the influence of cannabis and cocaine on cognitive impulse control and functional connectivity in the limbic 'reward' circuit because they experience a drug induced hyperdopaminergic state compared to individuals with high activity DBH genotypes (rs1611115 CC). Regular drug users (N = 122) received acute doses of cannabis (450 μg/kg THC), cocaine HCl 300 mg and placebo. Cognitive impulse control was assessed by means of the Matching Familiar Figures Test (MFFT). Resting state fMRI was measured in a subset of participants to determine functional connectivity between the nucleus accumbens (NAc) and (sub)cortical areas. The influence of cannabis and cocaine on impulsivity and functional connectivity significantly interacted with DBH genotype. Both drugs increased cognitive impulsivity in participants with CT/TT genotypes but not in CC participants. Both drugs also reduced functional connectivity between the NAc and the limbic lobe, prefrontal cortex, striatum and thalamus and primarily in individuals with CT/TT genotypes. Correlational analysis indicated a significant negative association between cognitive impulsivity and functional connectivity in subcortical areas of the brain. It is concluded that interference of cannabis and cocaine with cognitive impulse control and functional corticostriatal connectivity depends on DBH genotype. The present data provide a neural substrate and behavioral mechanism by which drug users can progress to drug seeking and may also offer a rationale for targeted pharmacotherapy in chronic drug users with high risk DBH genotypes.
Keywords: Cannabis; Cocaine; DBH genotype; Fuctional connectivity; Impulse control.
Conflict of interest statement
The authors report no financial interests or potential conflicts of interest. Informed consent All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
Figures


Similar articles
-
Single doses of THC and cocaine decrease proficiency of impulse control in heavy cannabis users.Br J Pharmacol. 2013 Dec;170(7):1410-20. doi: 10.1111/bph.12425. Br J Pharmacol. 2013. PMID: 24106872 Free PMC article. Clinical Trial.
-
Cannabis and tolerance: acute drug impairment as a function of cannabis use history.Sci Rep. 2016 May 26;6:26843. doi: 10.1038/srep26843. Sci Rep. 2016. PMID: 27225696 Free PMC article.
-
Understanding marijuana's effects on functional connectivity of the default mode network in patients with schizophrenia and co-occurring cannabis use disorder: A pilot investigation.Schizophr Res. 2018 Apr;194:70-77. doi: 10.1016/j.schres.2017.07.029. Epub 2017 Aug 18. Schizophr Res. 2018. PMID: 28823723 Free PMC article.
-
Brain Imaging Studies on the Cognitive, Pharmacological and Neurobiological Effects of Cannabis in Humans: Evidence from Studies of Adult Users.Curr Pharm Des. 2016;22(42):6366-6379. doi: 10.2174/1381612822666160822151323. Curr Pharm Des. 2016. PMID: 27549374 Review.
-
Effect of cocaine dependence on brain connections: clinical implications.Expert Rev Neurother. 2015;15(11):1307-19. doi: 10.1586/14737175.2015.1103183. Epub 2015 Oct 29. Expert Rev Neurother. 2015. PMID: 26512421 Free PMC article. Review.
Cited by
-
Interaction of Cannabis Use Disorder and Striatal Connectivity in Antipsychotic Treatment Response.Schizophr Bull Open. 2020 Jan;1(1):sgaa014. doi: 10.1093/schizbullopen/sgaa014. Epub 2020 Mar 20. Schizophr Bull Open. 2020. PMID: 32803161 Free PMC article.
-
The neuropsychopharmacology of cannabis: A review of human imaging studies.Pharmacol Ther. 2019 Mar;195:132-161. doi: 10.1016/j.pharmthera.2018.10.006. Epub 2018 Oct 19. Pharmacol Ther. 2019. PMID: 30347211 Free PMC article. Review.
-
Correlation between Traits of Emotion-Based Impulsivity and Intrinsic Default-Mode Network Activity.Neural Plast. 2017;2017:9297621. doi: 10.1155/2017/9297621. Epub 2017 Oct 31. Neural Plast. 2017. PMID: 29225975 Free PMC article.
-
Pharmacokinetic, behavioral, and brain activity effects of Δ9-tetrahydrocannabinol in adolescent male and female rats.Neuropsychopharmacology. 2021 Apr;46(5):959-969. doi: 10.1038/s41386-020-00839-w. Epub 2020 Sep 14. Neuropsychopharmacology. 2021. PMID: 32927465 Free PMC article.
-
Individual and combined effects of cannabidiol and Δ9-tetrahydrocannabinol on striato-cortical connectivity in the human brain.J Psychopharmacol. 2022 Jun;36(6):732-744. doi: 10.1177/02698811221092506. Epub 2022 May 20. J Psychopharmacol. 2022. PMID: 35596578 Free PMC article.
References
-
- Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, van Gerven JM, Ramsey NF, Lammertsma AA, Kahn RS. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology: Official publication of the American College of Neuropsychopharmacology. 2009;34:759–766. doi: 10.1038/npp.2008.138. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

