Mannose-Capped Lipoarabinomannan from Mycobacterium tuberculosis Induces CD4+ T Cell Anergy via GRAIL
- PMID: 26667170
- PMCID: PMC4707121
- DOI: 10.4049/jimmunol.1500710
Mannose-Capped Lipoarabinomannan from Mycobacterium tuberculosis Induces CD4+ T Cell Anergy via GRAIL
Abstract
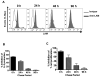
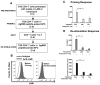
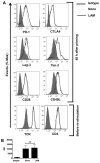
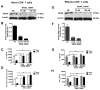
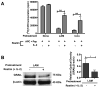
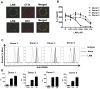
Mycobacterium tuberculosis cell wall glycolipid, lipoarabinomannan, can inhibit CD4(+) T cell activation by downregulating the phosphorylation of key proximal TCR signaling molecules: Lck, CD3ζ, ZAP70, and LAT. Inhibition of proximal TCR signaling can result in T cell anergy, in which T cells are inactivated following an Ag encounter, yet remain viable and hyporesponsive. We tested whether mannose-capped lipoarabinomannan (LAM)-induced inhibition of CD4(+) T cell activation resulted in CD4(+) T cell anergy. The presence of LAM during primary stimulation of P25 TCR-transgenic murine CD4(+) T cells with M. tuberculosis Ag85B peptide resulted in decreased proliferation and IL-2 production. P25 TCR-transgenic CD4(+) T cells primed in the presence of LAM also exhibited decreased response upon restimulation with Ag85B. The T cell anergic state persisted after the removal of LAM. Hyporesponsiveness to restimulation was not due to apoptosis, generation of Foxp3-positive regulatory T cells, or inhibitory cytokines. Acquisition of the anergic phenotype correlated with upregulation of gene related to anergy in lymphocytes (GRAIL) protein in CD4(+) T cells. Inhibition of human CD4(+) T cell activation by LAM also was associated with increased GRAIL expression. Small interfering RNA-mediated knockdown of GRAIL before LAM treatment abrogated LAM-induced hyporesponsiveness. In addition, exogenous IL-2 reversed defective proliferation by downregulating GRAIL expression. These results demonstrate that LAM upregulates GRAIL to induce anergy in Ag-reactive CD4(+) T cells. Induction of CD4(+) T cell anergy by LAM may represent one mechanism by which M. tuberculosis evades T cell recognition.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures

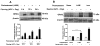
Similar articles
-
Mycobacterium tuberculosis Membrane Vesicles Inhibit T Cell Activation.J Immunol. 2017 Mar 1;198(5):2028-2037. doi: 10.4049/jimmunol.1601199. Epub 2017 Jan 25. J Immunol. 2017. PMID: 28122965 Free PMC article.
-
Gene related to anergy in lymphocytes (GRAIL) expression in CD4+ T cells impairs actin cytoskeletal organization during T cell/antigen-presenting cell interactions.J Biol Chem. 2009 Dec 11;284(50):34674-81. doi: 10.1074/jbc.M109.024497. Epub 2009 Oct 15. J Biol Chem. 2009. PMID: 19833735 Free PMC article.
-
The avidity spectrum of T cell receptor interactions accounts for T cell anergy in a double transgenic model.J Exp Med. 1999 Jan 18;189(2):265-78. doi: 10.1084/jem.189.2.265. J Exp Med. 1999. PMID: 9892609 Free PMC article.
-
Molecular mechanisms of CD4+ T-cell anergy.Nat Rev Immunol. 2007 Aug;7(8):599-609. doi: 10.1038/nri2131. Epub 2007 Jul 6. Nat Rev Immunol. 2007. PMID: 17612584 Review.
-
Lipoarabinomannan in Active and Passive Protection Against Tuberculosis.Front Immunol. 2019 Sep 11;10:1968. doi: 10.3389/fimmu.2019.01968. eCollection 2019. Front Immunol. 2019. PMID: 31572351 Free PMC article. Review.
Cited by
-
Immunological hyporesponsiveness in tuberculosis: The role of mycobacterial glycolipids.Front Immunol. 2022 Dec 2;13:1035122. doi: 10.3389/fimmu.2022.1035122. eCollection 2022. Front Immunol. 2022. PMID: 36544778 Free PMC article. Review.
-
Finding and filling the knowledge gaps in mechanisms of T cell-mediated TB immunity to inform vaccine design.Nat Rev Immunol. 2025 Jun 13. doi: 10.1038/s41577-025-01192-z. Online ahead of print. Nat Rev Immunol. 2025. PMID: 40514544 Review.
-
In-depth systems biological evaluation of bovine alveolar macrophages suggests novel insights into molecular mechanisms underlying Mycobacterium bovis infection.Front Microbiol. 2022 Nov 30;13:1041314. doi: 10.3389/fmicb.2022.1041314. eCollection 2022. Front Microbiol. 2022. Retraction in: Front Microbiol. 2025 Apr 10;16:1605607. doi: 10.3389/fmicb.2025.1605607. PMID: 36532492 Free PMC article. Retracted.
-
Mycobacterial mannose-capped lipoarabinomannan: a modulator bridging innate and adaptive immunity.Emerg Microbes Infect. 2019;8(1):1168-1177. doi: 10.1080/22221751.2019.1649097. Emerg Microbes Infect. 2019. PMID: 31379262 Free PMC article. Review.
-
Mycobacterium tuberculosis Membrane Vesicles Inhibit T Cell Activation.J Immunol. 2017 Mar 1;198(5):2028-2037. doi: 10.4049/jimmunol.1601199. Epub 2017 Jan 25. J Immunol. 2017. PMID: 28122965 Free PMC article.
References
-
- Commandeur S, van Meijgaarden KE, Prins C, Pichugin AV, Dijkman K, van den Eeden SJ, Friggen AH, Franken KL, Dolganov G, Kramnik I, Schoolnik GK, Oftung F, Korsvold GE, Geluk A, Ottenhoff TH. An unbiased genome-wide Mycobacterium tuberculosis gene expression approach to discover antigens targeted by human T cells expressed during pulmonary infection. J immunol. 2013;190:1659–1671. - PubMed
-
- Lindestam Arlehamn CS, Gerasimova A, Mele F, Henderson R, Swann J, Greenbaum JA, Kim Y, Sidney J, James EA, Taplitz R, McKinney DM, Kwok WW, Grey H, Sallusto F, Peters B, Sette A. Memory T cells in latent Mycobacterium tuberculosis infection are directed against three antigenic islands and largely contained in a CXCR3+CCR6+ Th1 subset. PLoS Pathogens. 2013;9:e1003130. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI027243/AI/NIAID NIH HHS/United States
- HL055967/HL/NHLBI NIH HHS/United States
- D43-TW009780/TW/FIC NIH HHS/United States
- D43 TW000011/TW/FIC NIH HHS/United States
- N01 AI070022/AI/NIAID NIH HHS/United States
- R01 HL055967/HL/NHLBI NIH HHS/United States
- P30 CA043703/CA/NCI NIH HHS/United States
- D43-TW000011/TW/FIC NIH HHS/United States
- D43 TW009780/TW/FIC NIH HHS/United States
- N01-AI-70022/AI/NIAID NIH HHS/United States
- R21 AI099494/AI/NIAID NIH HHS/United States
- R01 AI034343/AI/NIAID NIH HHS/United States
- R01 AI027243/AI/NIAID NIH HHS/United States
- R01 AI069085/AI/NIAID NIH HHS/United States
- HHSN266200700022C/PHS HHS/United States
- R01 HL106798/HL/NHLBI NIH HHS/United States
- P30 AI036219/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

