Evaluation of humoral immune status in porcine epidemic diarrhea virus (PEDV) infected sows under field conditions
- PMID: 26667229
- PMCID: PMC4699368
- DOI: 10.1186/s13567-015-0285-x
Evaluation of humoral immune status in porcine epidemic diarrhea virus (PEDV) infected sows under field conditions
Abstract
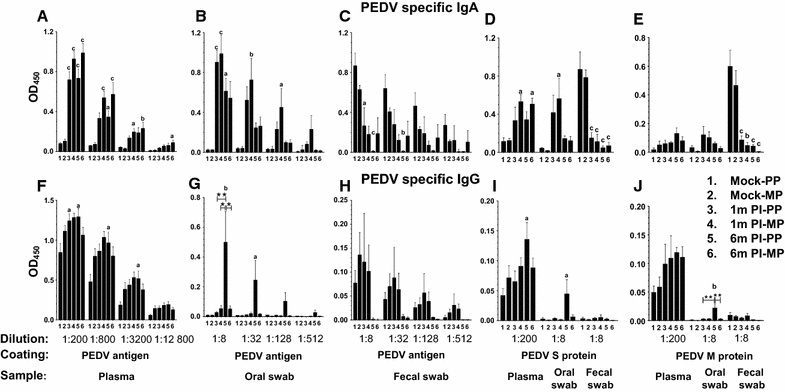
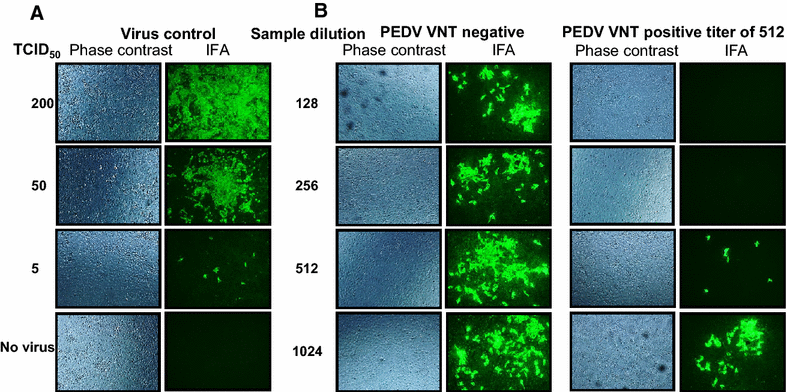
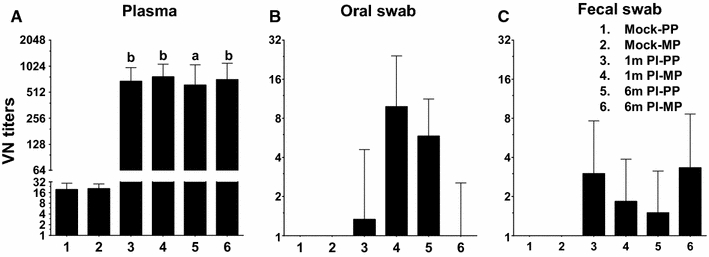
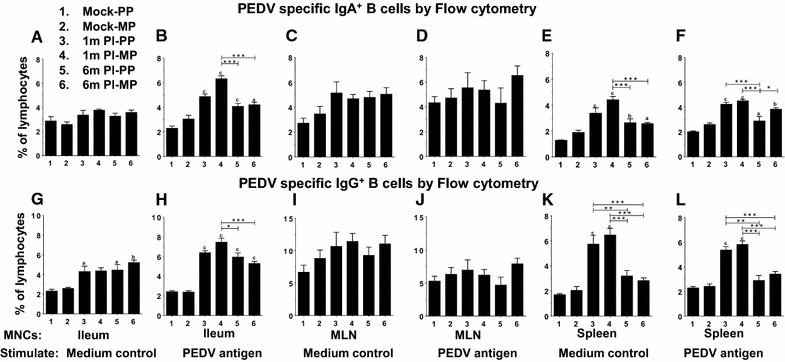
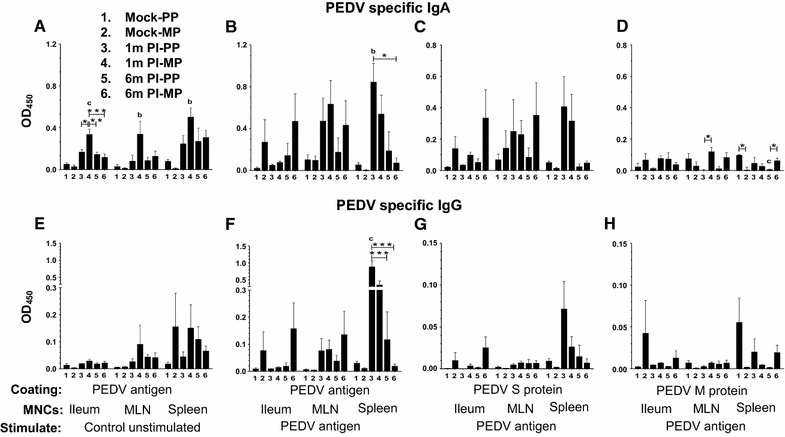
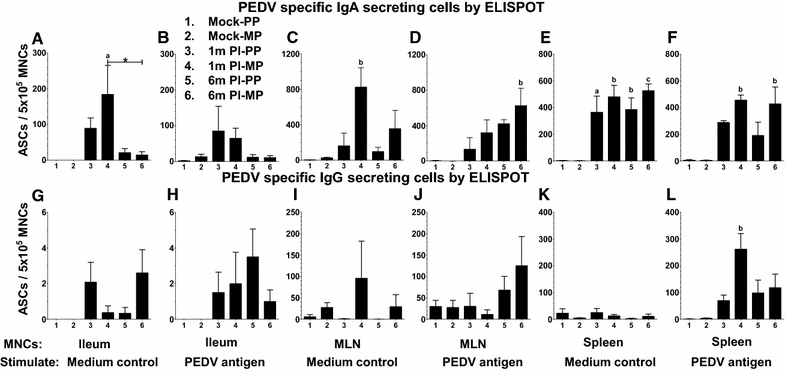
Porcine epidemic diarrhea virus (PEDV) is an economically devastating enteric disease in the swine industry. The virus infects pigs of all ages, but it cause severe clinical disease in neonatal suckling pigs with up to 100% mortality. Currently, available vaccines are not completely effective and feedback methods utilizing PEDV infected material has variable success in preventing reinfection. Comprehensive information on the levels and duration of effector/memory IgA and IgG antibody secreting B cell response in the intestines and lymphoid organs of PEDV-infected sows, and their association with specific antibody levels in clinical samples such as plasma, oral fluid, and feces is important. Therefore, our goal in this study was to quantify PEDV specific IgA and IgG B cell responses in sows at approximately 1 and 6 months post-infection in commercial swine herds, including parity one and higher sows. Our data indicated that evaluation of both PEDV specific IgA and IgG antibody levels in the plasma and oral fluid (but not feces) samples is beneficial in disease diagnosis. PEDV specific B cell response in the intestine and spleen of infected sows decline by 6 months, and this associates with specific antibody levels in the plasma and oral fluid samples; but the virus neutralization titers in plasma remains high beyond 6 months post-infection. In conclusion, in sows infected with PEDV the presence of effector/memory B cell response and strong virus neutralization titers in plasma up to 6 months post-infection, suggests their potential to protect sows from reinfection and provide maternal immunity to neonates, but challenge studies are required to confirm such responses.
Figures
Similar articles
-
Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): Historical and current concepts.Virus Res. 2016 Dec 2;226:93-107. doi: 10.1016/j.virusres.2016.05.016. Epub 2016 May 19. Virus Res. 2016. PMID: 27212686 Free PMC article. Review.
-
Humoral immune responses in piglets experimentally infected with a field strain of porcine epidemic diarrhea virus.Vet Microbiol. 2020 Jul;246:108742. doi: 10.1016/j.vetmic.2020.108742. Epub 2020 May 31. Vet Microbiol. 2020. PMID: 32605747
-
Systemic and intestinal porcine epidemic diarrhea virus-specific antibody response and distribution of antibody-secreting cells in experimentally infected conventional pigs.Vet Res. 2021 Jan 4;52(1):2. doi: 10.1186/s13567-020-00880-z. Vet Res. 2021. PMID: 33397461 Free PMC article.
-
Stage of Gestation at Porcine Epidemic Diarrhea Virus Infection of Pregnant Swine Impacts Maternal Immunity and Lactogenic Immune Protection of Neonatal Suckling Piglets.Front Immunol. 2019 Apr 24;10:727. doi: 10.3389/fimmu.2019.00727. eCollection 2019. Front Immunol. 2019. PMID: 31068924 Free PMC article.
-
Status of vaccines for porcine epidemic diarrhea virus in the United States and Canada.Virus Res. 2016 Dec 2;226:108-116. doi: 10.1016/j.virusres.2016.08.005. Epub 2016 Aug 18. Virus Res. 2016. PMID: 27545066 Review.
Cited by
-
Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): Historical and current concepts.Virus Res. 2016 Dec 2;226:93-107. doi: 10.1016/j.virusres.2016.05.016. Epub 2016 May 19. Virus Res. 2016. PMID: 27212686 Free PMC article. Review.
-
Isolation, Identification, and Evaluation of the Pathogenicity of a Porcine Enterovirus G Isolated From China.Front Vet Sci. 2021 Jul 22;8:712679. doi: 10.3389/fvets.2021.712679. eCollection 2021. Front Vet Sci. 2021. PMID: 34368288 Free PMC article.
-
Porcine epidemic diarrhea virus: An overview of current virological and serological diagnostic methods.Virus Res. 2016 Dec 2;226:60-70. doi: 10.1016/j.virusres.2016.05.013. Epub 2016 May 14. Virus Res. 2016. PMID: 27189041 Free PMC article. Review.
-
Development of indirect enzyme-linked immunosorbent assay for detection of porcine epidemic diarrhea virus specific antibodies (IgG) in serum of naturally infected pigs.BMC Vet Res. 2019 Nov 12;15(1):409. doi: 10.1186/s12917-019-2123-2. BMC Vet Res. 2019. PMID: 31718620 Free PMC article.
-
Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control.Virus Res. 2020 Sep;286:198045. doi: 10.1016/j.virusres.2020.198045. Epub 2020 Jun 2. Virus Res. 2020. PMID: 32502552 Free PMC article. Review.
References
-
- Stevenson GW, Hoang H, Schwartz KJ, Burrough ER, Sun D, Madson D, Cooper VL, Pillatzki A, Gauger P, Schmitt BJ, Koster LG, Killian ML, Yoon KJ. Emergence of porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J Vet Diagn Invest. 2013;25:649–654. doi: 10.1177/1040638713501675. - DOI - PubMed
-
- The pig site database (2015) http://www.thepigsite.com/swinenews/36126/ped-update-in-us-27-states-aff... Accessed 23 Nov 2015
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

