Biosynthesis and Reactivity of Cysteine Persulfides in Signaling
- PMID: 26667407
- PMCID: PMC4795164
- DOI: 10.1021/jacs.5b10494
Biosynthesis and Reactivity of Cysteine Persulfides in Signaling
Abstract
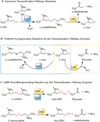
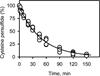
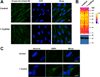
Hydrogen sulfide (H2S) elicits pleiotropic physiological effects ranging from modulation of cardiovascular to CNS functions. A dominant method for transmission of sulfide-based signals is via posttranslational modification of reactive cysteine thiols to persulfides. However, the source of the persulfide donor and whether its relationship to H2S is as a product or precursor is controversial. The transsulfuration pathway enzymes can synthesize cysteine persulfide (Cys-SSH) from cystine and H2S from cysteine and/or homocysteine. Recently, Cys-SSH was proposed as the primary product of the transsulfuration pathway with H2S representing a decomposition product of Cys-SSH. Our detailed kinetic analyses demonstrate a robust capacity for Cys-SSH production by the human transsulfuration pathway enzymes, cystathionine beta-synthase and γ-cystathionase (CSE) and for homocysteine persulfide synthesis from homocystine by CSE only. However, in the reducing cytoplasmic milieu where the concentration of reduced thiols is significantly higher than of disulfides, substrate level regulation favors the synthesis of H2S over persulfides. Mathematical modeling at physiologically relevant hepatic substrate concentrations predicts that H2S rather than Cys-SSH is the primary product of the transsulfuration enzymes with CSE being the dominant producer. The half-life of the metastable Cys-SSH product is short and decomposition leads to a mixture of polysulfides (Cys-S-(S)n-S-Cys). These in vitro data, together with the intrinsic reactivity of Cys-SSH for cysteinyl versus sulfur transfer, are consistent with the absence of an observable increase in protein persulfidation in cells in response to exogenous cystine and evidence for the formation of polysulfides under these conditions.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

