The C-Type Lectin Receptor MCL Mediates Vaccine-Induced Immunity against Infection with Blastomyces dermatitidis
- PMID: 26667836
- PMCID: PMC4771354
- DOI: 10.1128/IAI.01263-15
The C-Type Lectin Receptor MCL Mediates Vaccine-Induced Immunity against Infection with Blastomyces dermatitidis
Abstract
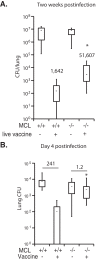
C-type lectin receptors (CLRs) are essential in shaping the immune response to fungal pathogens. Vaccine-induced resistance requires Dectin-2 to promote differentiation of antifungal Th1 and Th17 cells. Since Dectin-2 and MCL heterodimerize and both CLRs use FcRγ as the signaling adaptor, we investigated the role of MCL in vaccine immunity to the fungal pathogen Blastomyces dermatitidis. MCL(-/-) mice showed impaired vaccine resistance against B. dermatitidis infection compared to that of wild-type animals. The lack of resistance correlated with the reduced recruitment of Th17 cells to the lung upon recall following experimental challenge and impaired interleukin-17 (IL-17) production by vaccine antigen-stimulated splenocytes in vitro. Soluble MCL fusion protein recognized and bound a water-soluble ligand from the cell wall of vaccine yeast, but the addition of soluble Dectin-2 fusion protein did not augment ligand recognition by MCL. Taken together, our data indicate that MCL regulates the development of vaccine-induced Th17 cells and protective immunity against lethal experimental infection with B. dermatitidis.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Schmidt CS, White CJ, Ibrahim AS, Filler SG, Fu Y, Yeaman MR, Edwards JE Jr, Hennessey JP Jr. 2012. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine 30:7594–7600. doi:10.1016/j.vaccine.2012.10.038. - DOI - PMC - PubMed
-
- Wüthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, Cole G, Klein B. 2011. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Investig 121:554–568. doi:10.1172/JCI43984. - DOI - PMC - PubMed
-
- Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE Jr, Spellberg B. 2009. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog 5:e1000703. doi:10.1371/journal.ppat.1000703. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

