Validating Early Post-Transplant Outcomes Reported for Recipients of Deceased Donor Kidney Transplants
- PMID: 26668026
- PMCID: PMC4741043
- DOI: 10.2215/CJN.06950615
Validating Early Post-Transplant Outcomes Reported for Recipients of Deceased Donor Kidney Transplants
Abstract
Background and objectives: Data reported to the Organ Procurement and Transplantation Network (OPTN) are used in kidney transplant research, policy development, and assessment of center quality, but the accuracy of early post-transplant outcome measures is unknown.
Design, setting, participants, & measurements: The Deceased Donor Study (DDS) is a prospective cohort study at five transplant centers. Research coordinators manually abstracted data from electronic records for 557 adults who underwent deceased donor kidney transplantation between April of 2010 and November of 2013. We compared the post-transplant outcomes of delayed graft function (DGF; defined as dialysis in the first post-transplant week), acute rejection, and post-transplant serum creatinine reported to the OPTN with data collected for the DDS.
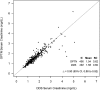
Results: Median kidney donor risk index was 1.22 (interquartile range [IQR], 0.97-1.53). Median recipient age was 55 (IQR, 46-63) years old, 63% were men, and 47% were black; 93% had received dialysis before transplant. Using DDS data as the gold standard, we found that pretransplant dialysis was not reported to the OPTN in only 11 (2%) instances. DGF in OPTN data had a sensitivity of 89% (95% confidence interval [95% CI], 84% to 93%) and specificity of 98% (95% CI, 96% to 99%). Surprisingly, the OPTN data accurately identified acute allograft rejection in only 20 of 47 instances (n=488; sensitivity of 43%; 95% CI, 17% to 73%). Across participating centers, sensitivity of acute rejection varied widely from 23% to 100%, whereas specificity was uniformly high (92%-100%). Six-month serum creatinine values in DDS and OPTN data had high concordance (n=490; Lin concordance correlation =0.90; 95% CI, 0.88 to 0.92).
Conclusions: OPTN outcomes for recipients of deceased donor kidney transplants have high validity for DGF and 6-month allograft function but lack sensitivity in detecting rejection. Future studies using OPTN data may consider focusing on allograft function at 6 months as a useful outcome.
Keywords: acute rejection; adult; allografts; deceased donors; delayed graft function; humans; kidney transplantation; tissue and organ procurement; tissue donors; validation studies.
Copyright © 2016 by the American Society of Nephrology.
Figures

Similar articles
-
Similar risk profiles for post-transplant renal dysfunction and long-term graft failure: UNOS/OPTN database analysis.Kidney Int. 2004 May;65(5):1906-13. doi: 10.1111/j.1523-1755.2004.00589.x. Kidney Int. 2004. PMID: 15086934
-
Predictors and outcomes of delayed graft function after living-donor kidney transplantation.Transpl Int. 2016 Jan;29(1):81-7. doi: 10.1111/tri.12696. Epub 2015 Oct 26. Transpl Int. 2016. PMID: 26432507
-
Donor positive blood culture is associated with delayed graft function in kidney transplant recipients: a propensity score analysis of the UNOS database.Clin Transplant. 2016 Apr;30(4):415-20. doi: 10.1111/ctr.12703. Epub 2016 Feb 24. Clin Transplant. 2016. PMID: 26840885
-
Delayed graft function and its management in children.Pediatr Nephrol. 2017 Jul;32(7):1157-1167. doi: 10.1007/s00467-016-3528-9. Epub 2016 Oct 24. Pediatr Nephrol. 2017. PMID: 27778091 Review.
-
Delayed Graft Function in Living-Donor Kidney Transplant: A Middle Eastern Perspective.Exp Clin Transplant. 2016 Feb;14(1):1-11. Exp Clin Transplant. 2016. PMID: 26862818 Review.
Cited by
-
Association of Deceased Donor Acute Kidney Injury With Recipient Graft Survival.JAMA Netw Open. 2020 Jan 3;3(1):e1918634. doi: 10.1001/jamanetworkopen.2019.18634. JAMA Netw Open. 2020. PMID: 31913491 Free PMC article.
-
Comparing Outcomes between Antibody Induction Therapies in Kidney Transplantation.J Am Soc Nephrol. 2017 Jul;28(7):2188-2200. doi: 10.1681/ASN.2016070768. Epub 2017 Mar 20. J Am Soc Nephrol. 2017. PMID: 28320767 Free PMC article.
-
Donor Urinary C5a Levels Independently Correlate With Posttransplant Delayed Graft Function.Transplantation. 2019 Jan;103(1):e29-e35. doi: 10.1097/TP.0000000000002494. Transplantation. 2019. PMID: 30451738 Free PMC article.
-
Therapeutic Donor Kidney Transplant Outcomes: Comparing Early US Experiences Using Optimal Matching.Transplant Direct. 2023 Nov 2;9(12):e1554. doi: 10.1097/TXD.0000000000001554. eCollection 2023 Dec. Transplant Direct. 2023. PMID: 37928484 Free PMC article.
-
Associations between Deceased-Donor Urine MCP-1 and Kidney Transplant Outcomes.Kidney Int Rep. 2017 Jul;2(4):749-758. doi: 10.1016/j.ekir.2017.03.007. Epub 2017 Mar 31. Kidney Int Rep. 2017. PMID: 28730184 Free PMC article.
References
-
- Dickinson DM, Bryant PC, Williams MC, Levine GN, Li S, Welch JC, Keck BM, Webb RL: Transplant data: Sources, collection, and caveats. Am J Transplant 4[Suppl 9]: 13–26, 2004 - PubMed
-
- Axelrod DA, Lentine KL, Xiao H, Bubolz T, Goodman D, Freeman R, Tuttle-Newhall JE, Schnitzler MA: Accountability for end-stage organ care: Implications of geographic variation in access to kidney transplantation. Surgery 155: 734–742, 2014 - PubMed
-
- Kuo HT, Sampaio MS, Vincenti F, Bunnapradist S: Associations of pretransplant diabetes mellitus, new-onset diabetes after transplant, and acute rejection with transplant outcomes: An analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) database. Am J Kidney Dis 56: 1127–1139, 2010 - PubMed
-
- Locke JE, James NT, Mannon RB, Mehta SG, Pappas PG, Baddley JW, Desai NM, Montgomery RA, Segev DL: Immunosuppression regimen and the risk of acute rejection in HIV-infected kidney transplant recipients. Transplantation 97: 446–450, 2014 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

