Rapid Response to Cyclosporin A and Favorable Renal Outcome in Nongenetic Versus Genetic Steroid-Resistant Nephrotic Syndrome
- PMID: 26668027
- PMCID: PMC4741047
- DOI: 10.2215/CJN.07370715
Rapid Response to Cyclosporin A and Favorable Renal Outcome in Nongenetic Versus Genetic Steroid-Resistant Nephrotic Syndrome
Abstract
Background and objectives: Treatment of congenital nephrotic syndrome (CNS) and steroid-resistant nephrotic syndrome (SRNS) is demanding, and renal prognosis is poor. Numerous causative gene mutations have been identified in SRNS that affect the renal podocyte. In the era of high-throughput sequencing techniques, patients with nongenetic SRNS frequently escape the scientific interest. We here present the long-term data of the German CNS/SRNS Follow-Up Study, focusing on the response to cyclosporin A (CsA) in patients with nongenetic versus genetic disease.
Design, setting, participants, & measurements: Cross-sectional and longitudinal clinical data were collected from 231 patients with CNS/SRNS treated at eight university pediatric nephrology units with a median observation time of 113 months (interquartile range, 50-178). Genotyping was performed systematically in all patients.
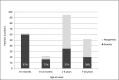
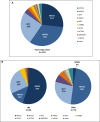
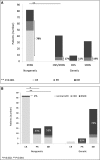
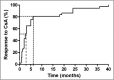
Results: The overall mutation detection rate was high at 57% (97% in CNS and 41% in SRNS); 85% of all mutations were identified by the analysis of three single genes only (NPHS1, NPHS2, and WT1), accounting for 92% of all mutations in patients with CNS and 79% of all mutations in patients with SRNS. Remission of the disease in nongenetic SRNS was observed in 78% of patients after a median treatment period of 2.5 months; 82% of nongenetic patients responded within 6 months of therapy, and 98% of patients with nongenetic SRNS and CsA-induced complete remission (normalbuminemia and no proteinuria) maintained a normal renal function. Genetic SRNS, on the contrary, is associated with a high rate of ESRD in 66% of patients. Only 3% of patients with genetic SRNS experienced a complete remission and 16% of patients with genetic SRNS experienced a partial remission after CsA therapy.
Conclusions: The efficacy of CsA is high in nonhereditary SRNS, with an excellent prognosis of renal function in the large majority of patients. CsA should be given for a minimum period of 6 months in these patients with nongenetic SRNS. In genetic SRNS, response to CsA was low and restricted to exceptional patients.
Keywords: FSGS; NPHS1; NPHS2; WT1; cyclosporine A; humans; kidney failure, chronic; mutation; steroid resistant nephrotic syndrome.
Copyright © 2016 by the American Society of Nephrology.
Figures
References
-
- Lombel RM, Hodson EM, Gipson DS, Kidney Disease: Improving Global Outcomes : Treatment of steroid-resistant nephrotic syndrome in children: New guidelines from KDIGO. Pediatr Nephrol 28: 409–414, 2013 - PubMed
-
- Cattran DC, Alexopoulos E, Heering P, Hoyer PF, Johnston A, Meyrier A, Ponticelli C, Saito T, Choukroun G, Nachman P, Praga M, Yoshikawa N: Cyclosporin in idiopathic glomerular disease associated with the nephrotic syndrome: Workshop recommendations. Kidney Int 72: 1429–1447, 2007 - PubMed
-
- Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL, North America Nephrotic Syndrome Study Group : A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. Kidney Int 56: 2220–2226, 1999 - PubMed
-
- Ponticelli C, Rizzoni G, Edefonti A, Altieri P, Rivolta E, Rinaldi S, Ghio L, Lusvarghi E, Gusmano R, Locatelli F, Pasquali S, Castellani A, Casa-Alberighi OD: A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int 43: 1377–1384, 1993 - PubMed
-
- Garin EH, Orak JK, Hiott KL, Sutherland SE: Cyclosporine therapy for steroid-resistant nephrotic syndrome. A controlled study. Am J Dis Child 142: 985–988, 1988 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

