Perturbation of Critical Prolines in Gloeobacter violaceus Ligand-gated Ion Channel (GLIC) Supports Conserved Gating Motions among Cys-loop Receptors
- PMID: 26668320
- PMCID: PMC4813548
- DOI: 10.1074/jbc.M115.694372
Perturbation of Critical Prolines in Gloeobacter violaceus Ligand-gated Ion Channel (GLIC) Supports Conserved Gating Motions among Cys-loop Receptors
Abstract
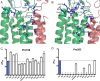
Gloeobacter violaceus ligand-gated ion channel (GLIC) has served as a valuable structural and functional model for the eukaryotic Cys-loop receptor superfamily. In Cys-loop and other receptors, we have previously demonstrated the crucial roles played by several conserved prolines. Here we explore the role of prolines in the gating transitions of GLIC. As conventional substitutions at some positions resulted in nonfunctional proteins, we used in vivo non-canonical amino acid mutagenesis to determine the specific structural requirements at these sites. Receptors were expressed heterologously in Xenopus laevis oocytes, and whole-cell electrophysiology was used to monitor channel activity. Pro-119 in the Cys-loop, Pro-198 and Pro-203 in the M1 helix, and Pro-299 in the M4 helix were sensitive to substitution, and distinct roles in receptor activity were revealed for each. In the context of the available structural data for GLIC, the behaviors of Pro-119, Pro-203, and Pro-299 mutants are consistent with earlier proline mutagenesis work. However, the Pro-198 site displays a unique phenotype that gives evidence of the importance of the region surrounding this residue for the correct functioning of GLIC.
Keywords: Cys-loop receptor; Gloeobacter violaceus; electrophysiology; mutagenesis; non-canonical amino acid; non-standard mutagenesis; nonsense suppression; proline analogs; protein conformation; structure-function.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- dacosta C. J. B., and Baenziger J. E. (2013) Gating of pentameric ligand-gated ion channels: structural insights and ambiguities. Structure 21, 1271–1283 - PubMed
-
- Nys M., Kesters D., and Ulens C. (2013) Structural insights into Cys-loop receptor function and ligand recognition. Biochem. Pharmacol. 86, 1042–1053 - PubMed
-
- Hassaine G., Deluz C., Grasso L., Wyss R., Tol M. B., Hovius R., Graff A., Stahlberg H., Tomizaki T., Desmyter A., Moreau C., Li X.-D., Poitevin F., Vogel H., and Nury H. (2014) X-ray structure of the mouse serotonin 5-HT3 receptor. Nature 512, 276–281 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

