Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma
- PMID: 26668346
- PMCID: PMC4872014
- DOI: 10.1200/JCO.2015.64.2710
Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma
Abstract
Purpose: To evaluate the efficacy and safety of high-dose, hypofractionated proton beam therapy for hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC).
Materials and methods: In this single-arm, phase II, multi-institutional study, 92 patients with biopsy-confirmed HCC or ICC, determined to be unresectable by multidisciplinary review, with a Child-Turcotte-Pugh score (CTP) of A or B, ECOG performance status of 0 to 2, no extrahepatic disease, and no prior radiation received 15 fractions of proton therapy to a maximum total dose of 67.5 Gy equivalent. Sample size was calculated to demonstrate > 80% local control (LC) defined by Response Evaluation Criteria in Solid Tumors (RECIST) 1.0 criteria at 2 years for HCC patients, with the parallel goal of obtaining acceptable precision for estimating outcomes for ICC.
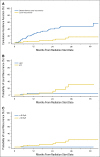
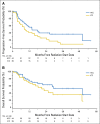
Results: Eighty-three patients were evaluable: 44 with HCC, 37 with ICC, and two with mixed HCC/ICC. The CTP score was A for 79.5% of patients and B for 15.7%; 4.8% of patients had no cirrhosis. Prior treatment had been given to 31.8% of HCC patients and 61.5% of ICC patients. The median maximum dimension was 5.0 cm (range, 1.9 to 12.0 cm) for HCC patients and 6.0 cm (range, 2.2 to 10.9 cm) for ICC patients. Multiple tumors were present in 27.3% of HCC patients and in 12.8% of ICC patients. Tumor vascular thrombosis was present in 29.5% of HCC patients and in 28.2% of ICC patients. The median dose delivered to both HCC and ICC patients was 58.0 Gy. With a median follow-up among survivors of 19.5 months, the LC rate at 2 years was 94.8% for HCC and 94.1% for ICC. The overall survival rate at 2 years was 63.2% for HCC and 46.5% ICC.
Conclusion: High-dose hypofractionated proton therapy demonstrated high LC rates for HCC and ICC safely, supporting ongoing phase III trials of radiation in HCC and ICC.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures
References
-
- Llovet JM, Ricci S, Mazzaferro V, et al. SHARP Investigators Study Group Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
-
- Valle J, Wasan H, Palmer DH, et al. ABC-02 Trial Investigators Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. - PubMed
-
- Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: Review of evidence and future opportunities. Int J Radiat Oncol Biol Phys. 2013;87:22–32. - PubMed
-
- Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. - PubMed
-
- Crane CH, Macdonald KO, Vauthey JN, et al. Limitations of conventional doses of chemoradiation for unresectable biliary cancer. Int J Radiat Oncol Biol Phys. 2002;53:969–974. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

