Recombination patterns in maize reveal limits to crossover homeostasis
- PMID: 26668366
- PMCID: PMC4703008
- DOI: 10.1073/pnas.1514265112
Recombination patterns in maize reveal limits to crossover homeostasis
Abstract
During meiotic recombination, double-strand breaks (DSBs) are formed in chromosomal DNA and then repaired as either crossovers (COs) or non-crossovers (NCOs). In most taxa, the number of DSBs vastly exceeds the number of COs. COs are required for generating genetic diversity in the progeny, as well as proper chromosome segregation. Their formation is tightly controlled so that there is at least one CO per pair of homologous chromosomes whereas the maximum number of COs per chromosome pair is fairly limited. One of the main mechanisms controlling the number of recombination events per meiosis is CO homeostasis, which maintains a stable CO number even when the DSB number is dramatically altered. The existence of CO homeostasis has been reported in several species, including mouse, yeast, and Caenorhabditis elegans. However, it is not known whether homeostasis exists in the same form in all species. In addition, the studies of homeostasis have been conducted using mutants and/or transgenic lines exhibiting fairly severe meiotic phenotypes, and it is unclear how important homeostasis is under normal physiological conditions. We found that, in maize, CO control is robust only to ensure one CO per chromosome pair. However, once this limit is reached, the CO number is linearly related to the DSB number. We propose that CO control is a multifaceted process whose different aspects have a varying degree of importance in different species.
Keywords: crossing-over; crossover homeostasis; double-strand breaks; meiosis; recombination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
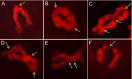
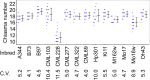
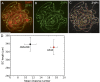
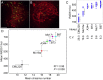

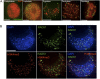
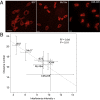
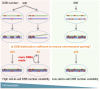
References
-
- Keeney S, Giroux CN, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88(3):375–384. - PubMed
-
- Borde V. The multiple roles of the Mre11 complex for meiotic recombination. Chromosome Res. 2007;15(5):551–563. - PubMed
-
- Hunter N, Kleckner N. The single-end invasion: An asymmetric intermediate at the double-strand break to double-holliday junction transition of meiotic recombination. Cell. 2001;106(1):59–70. - PubMed
-
- Bishop DK, Zickler D. Early decision: Meiotic crossover interference prior to stable strand exchange and synapsis. Cell. 2004;117(1):9–15. - PubMed
-
- Börner GV, Kleckner N, Hunter N. Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell. 2004;117(1):29–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

