Unveiling the crucial intermediates in androgen production
- PMID: 26668369
- PMCID: PMC4703009
- DOI: 10.1073/pnas.1519376113
Unveiling the crucial intermediates in androgen production
Abstract
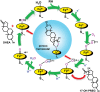
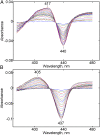
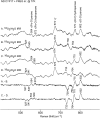
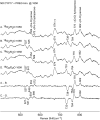
Ablation of androgen production through surgery is one strategy against prostate cancer, with the current focus placed on pharmaceutical intervention to restrict androgen synthesis selectively, an endeavor that could benefit from the enhanced understanding of enzymatic mechanisms that derives from characterization of key reaction intermediates. The multifunctional cytochrome P450 17A1 (CYP17A1) first catalyzes the typical hydroxylation of its primary substrate, pregnenolone (PREG) and then also orchestrates a remarkable C17-C20 bond cleavage (lyase) reaction, converting the 17-hydroxypregnenolone initial product to dehydroepiandrosterone, a process representing the first committed step in the biosynthesis of androgens. Now, we report the capture and structural characterization of intermediates produced during this lyase step: an initial peroxo-anion intermediate, poised for nucleophilic attack on the C20 position by a substrate-associated H-bond, and the crucial ferric peroxo-hemiacetal intermediate that precedes carbon-carbon (C-C) bond cleavage. These studies provide a rare glimpse at the actual structural determinants of a chemical transformation that carries profound physiological consequences.
Keywords: cytochrome P450; nanodiscs; peroxo-hemiacetal; resonance Raman spectroscopy; steroids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





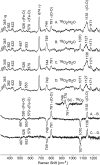

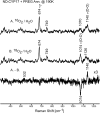

References
-
- Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;168(1):9–12. - PubMed
-
- Porubek D. CYP17A1: A biochemistry, chemistry, and clinical review. Curr Top Med Chem. 2013;13(12):1364–1384. - PubMed
-
- Ortiz de Montellano PR, editor. Cytochrome P450 Structure, Mechanism and Biochemistry. 3rd Ed Kluwer/Plenum; New York: 2005.
-
- Hrycay EG, Bandiera SM, editors. Advances in Experimental Medicine and Biology. Vol 851 Springer; London: 2015. Monooxygenase, Peroxidase and Peroxygenase Properties and Mechanisms of Cytochrome P450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

