Exogenous Hsp70 delays senescence and improves cognitive function in aging mice
- PMID: 26668376
- PMCID: PMC4702952
- DOI: 10.1073/pnas.1516131112
Exogenous Hsp70 delays senescence and improves cognitive function in aging mice
Abstract
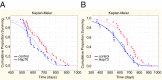
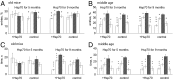
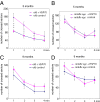
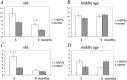
Molecular chaperone Heat Shock Protein 70 (Hsp70) plays an important protective role in various neurodegenerative disorders often associated with aging, but its activity and availability in neuronal tissue decrease with age. Here we explored the effects of intranasal administration of exogenous recombinant human Hsp70 (eHsp70) on lifespan and neurological parameters in middle-aged and old mice. Long-term administration of eHsp70 significantly enhanced the lifespan of animals of different age groups. Behavioral assessment after 5 and 9 mo of chronic eHsp70 administration demonstrated improved learning and memory in old mice. Likewise, the investigation of locomotor and exploratory activities after eHsp70 treatment demonstrated a significant therapeutic effect of this chaperone. Measurements of synaptophysin show that eHsp70 treatment in old mice resulted in larger synaptophysin-immunopositive areas and higher neuron density compared with control animals. Furthermore, eHsp70 treatment decreased accumulation of lipofuscin, an aging-related marker, in the brain and enhanced proteasome activity. The potential of eHsp70 intranasal treatment to protect synaptic machinery in old animals offers a unique pharmacological approach for various neurodegenerative disorders associated with human aging.
Keywords: Hsp70; aging; memory; proteasome; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

