Frequency preference and attention effects across cortical depths in the human primary auditory cortex
- PMID: 26668397
- PMCID: PMC4702984
- DOI: 10.1073/pnas.1507552112
Frequency preference and attention effects across cortical depths in the human primary auditory cortex
Abstract
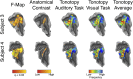
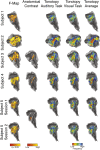
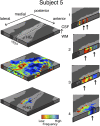
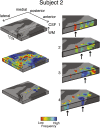
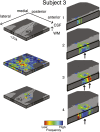
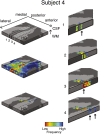
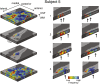
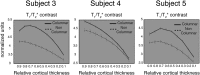
Columnar arrangements of neurons with similar preference have been suggested as the fundamental processing units of the cerebral cortex. Within these columnar arrangements, feed-forward information enters at middle cortical layers whereas feedback information arrives at superficial and deep layers. This interplay of feed-forward and feedback processing is at the core of perception and behavior. Here we provide in vivo evidence consistent with a columnar organization of the processing of sound frequency in the human auditory cortex. We measure submillimeter functional responses to sound frequency sweeps at high magnetic fields (7 tesla) and show that frequency preference is stable through cortical depth in primary auditory cortex. Furthermore, we demonstrate that-in this highly columnar cortex-task demands sharpen the frequency tuning in superficial cortical layers more than in middle or deep layers. These findings are pivotal to understanding mechanisms of neural information processing and flow during the active perception of sounds.
Keywords: 7-Tesla; columns; depth-dependent; fMRI; tonotopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Merzenich MM, Reid MD. Representation of the cochlea within the inferior colliculus of the cat. Brain Res. 1974;77(3):397–415. - PubMed
-
- Merzenich MM, Brugge JF. Representation of the cochlear partition of the superior temporal plane of the macaque monkey. Brain Res. 1973;50(2):275–296. - PubMed
-
- Formisano E, et al. Mirror-symmetric tonotopic maps in human primary auditory cortex. Neuron. 2003;40(4):859–869. - PubMed
-
- Winer JA, Schreiner CE, editors. The Auditory Cortex. Springer; New York: 2009.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

