Peroxisome proliferator-activated receptor-delta agonist ameliorated inflammasome activation in nonalcoholic fatty liver disease
- PMID: 26668503
- PMCID: PMC4671034
- DOI: 10.3748/wjg.v21.i45.12787
Peroxisome proliferator-activated receptor-delta agonist ameliorated inflammasome activation in nonalcoholic fatty liver disease
Abstract
Aim: To evaluate the inflammasome activation and the effect of peroxisome proliferator-activated receptors (PPAR)-δ agonist treatment in nonalcoholic fatty liver disease (NAFLD) models.
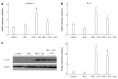
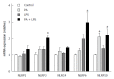
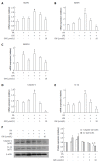
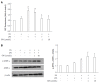
Methods: Male C57BL/6J mice were classified according to control or high fat diet (HFD) with or without PPAR-δ agonist (GW) over period of 12 wk [control, HFD, HFD + lipopolysaccharide (LPS), HFD + LPS + GW group]. HepG2 cells were exposed to palmitic acid (PA) and/or LPS in the absence or presence of GW.
Results: HFD caused glucose intolerance and hepatic steatosis. In mice fed an HFD with LPS, caspase-1 and interleukin (IL)-1β in the liver were significantly increased. Treatment with GW ameliorated the steatosis and inhibited overexpression of pro-inflammatory cytokines. In HepG2 cells, PA and LPS treatment markedly increased mRNA of several nucleotide-binding and oligomerization domain-like receptor family members (NLRP3, NLRP6, and NLRP10), caspase-1 and IL-1β. PA and LPS also exaggerated reactive oxygen species production. All of the above effects of PA and LPS were reduced by GW. GW also enhanced the phosphorylation of AMPK-α.
Conclusion: PPAR-δ agonist reduces fatty acid-induced inflammation and steatosis by suppressing inflammasome activation. Targeting the inflammasome by the PPAR-δ agonist may have therapeutic implication for NAFLD.
Keywords: Inflammasome; Nonalcoholic fatty liver disease; Nonalcoholic steatohepatitis; Nucleotide-binding and oligomerization domain-like receptor; Peroxisome proliferator-activated receptors delta.
Figures



Similar articles
-
Dietary saturated fatty acid and polyunsaturated fatty acid oppositely affect hepatic NOD-like receptor protein 3 inflammasome through regulating nuclear factor-kappa B activation.World J Gastroenterol. 2016 Feb 28;22(8):2533-44. doi: 10.3748/wjg.v22.i8.2533. World J Gastroenterol. 2016. PMID: 26937141 Free PMC article.
-
PPAR δ inhibition protects against palmitic acid-LPS induced lipidosis and injury in cultured hepatocyte L02 cell.Int J Med Sci. 2019 Oct 21;16(12):1593-1603. doi: 10.7150/ijms.37677. eCollection 2019. Int J Med Sci. 2019. PMID: 31839747 Free PMC article.
-
Leonurus japonicus Houtt Attenuates Nonalcoholic Fatty Liver Disease in Free Fatty Acid-Induced HepG2 Cells and Mice Fed a High-Fat Diet.Nutrients. 2017 Dec 25;10(1):20. doi: 10.3390/nu10010020. Nutrients. 2017. PMID: 29295591 Free PMC article.
-
Novel insights into the mechanisms whereby isoflavones protect against fatty liver disease.World J Gastroenterol. 2015 Jan 28;21(4):1099-107. doi: 10.3748/wjg.v21.i4.1099. World J Gastroenterol. 2015. PMID: 25632182 Free PMC article. Review.
-
Revealing the role of peroxisome proliferator-activated receptor β/δ in nonalcoholic fatty liver disease.Metabolism. 2021 Jan;114:154342. doi: 10.1016/j.metabol.2020.154342. Epub 2020 Aug 15. Metabolism. 2021. PMID: 32810487 Review.
Cited by
-
The role of the interleukin family in liver fibrosis.Front Immunol. 2025 Feb 10;16:1497095. doi: 10.3389/fimmu.2025.1497095. eCollection 2025. Front Immunol. 2025. PMID: 39995661 Free PMC article. Review.
-
Significance of elevated serum and hepatic NOD-like receptor pyrin domain containing 3 (NLRP3) in hepatitis C virus-related liver disease.Sci Rep. 2022 Nov 14;12(1):19528. doi: 10.1038/s41598-022-22022-5. Sci Rep. 2022. PMID: 36376416 Free PMC article.
-
Natural Drugs: A New Direction for the Prevention and Treatment of Diabetes.Molecules. 2023 Jul 20;28(14):5525. doi: 10.3390/molecules28145525. Molecules. 2023. PMID: 37513397 Free PMC article. Review.
-
IL-1 Family Cytokine Pathways Underlying NAFLD: Towards New Treatment Strategies.Trends Mol Med. 2018 May;24(5):458-471. doi: 10.1016/j.molmed.2018.03.005. Epub 2018 Apr 14. Trends Mol Med. 2018. PMID: 29665983 Free PMC article. Review.
-
Characterisation of extracellular vesicles isolated from hydatid cyst fluid and evaluation of immunomodulatory effects on human monocytes.J Cell Mol Med. 2023 Sep;27(17):2614-2625. doi: 10.1111/jcmm.17894. Epub 2023 Aug 2. J Cell Mol Med. 2023. PMID: 37530547 Free PMC article.
References
-
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. - PubMed
-
- Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

