Pervasive satellite cell contribution to uninjured adult muscle fibers
- PMID: 26668715
- PMCID: PMC4677447
- DOI: 10.1186/s13395-015-0067-1
Pervasive satellite cell contribution to uninjured adult muscle fibers
Abstract
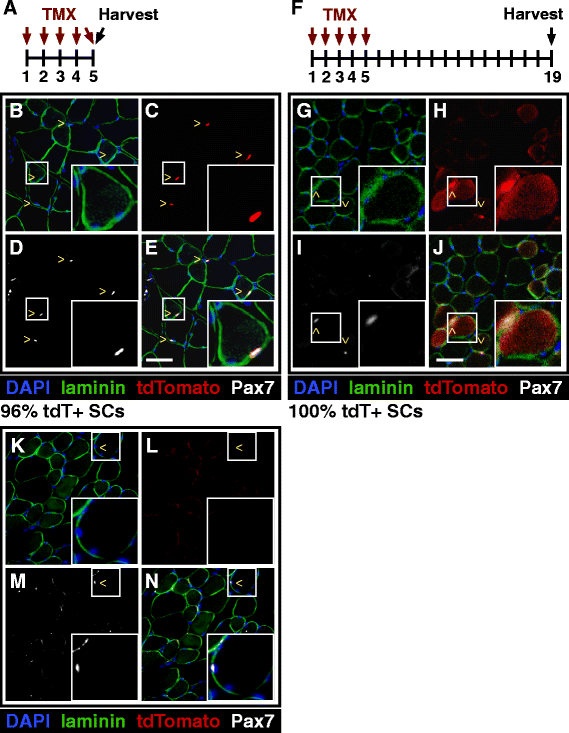
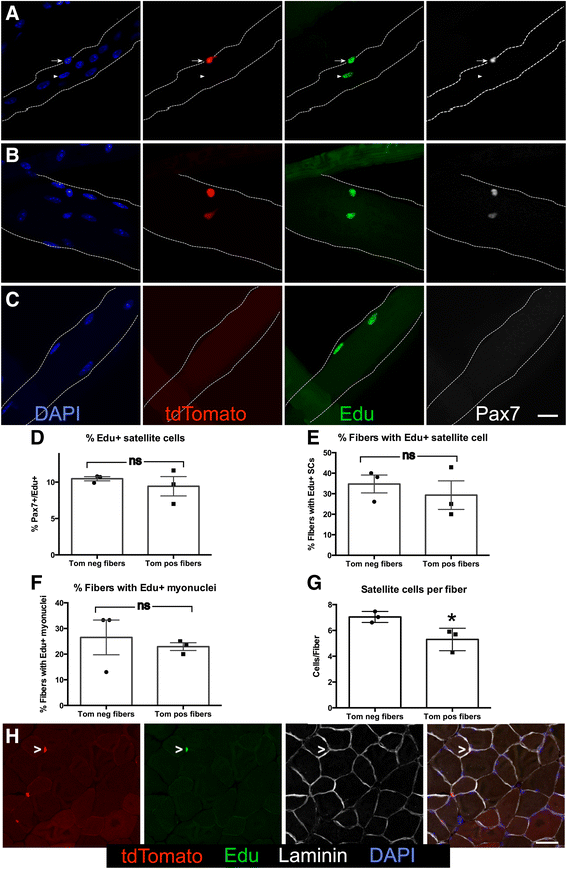
Background: Adult skeletal muscle adapts to functional needs, maintaining consistent numbers of myonuclei and stem cells. Although resident muscle stem cells or satellite cells are required for muscle growth and repair, in uninjured muscle, these cells appear quiescent and metabolically inactive. To investigate the satellite cell contribution to myofibers in adult uninjured skeletal muscle, we labeled satellite cells by inducing a recombination of LSL-tdTomato in Pax7(CreER) mice and scoring tdTomato+ myofibers as an indicator of satellite cell fusion.
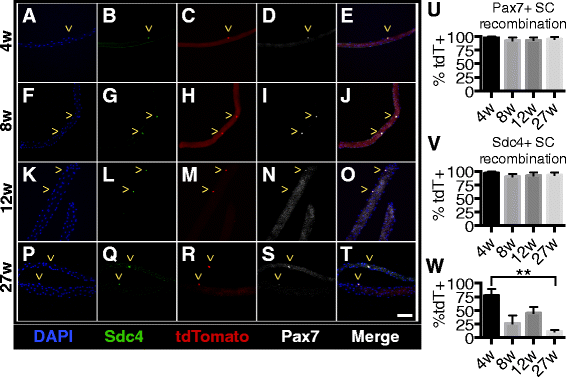
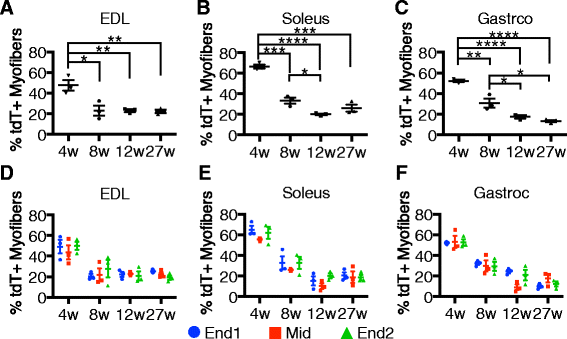
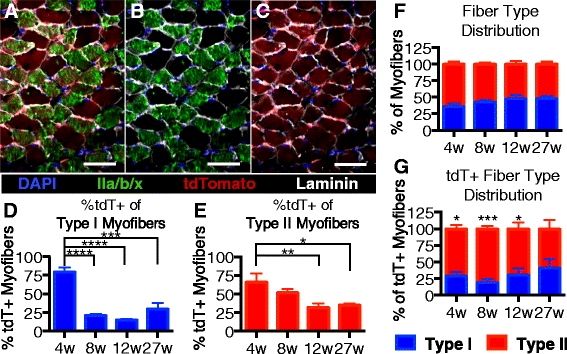
Results: Satellite cell fusion into myofibers plateaus postnatally between 8 and 12 weeks of age, reaching a steady state in hindlimb muscles, but in extra ocular or diaphragm muscles, satellite cell fusion is maintained at postnatal levels irrespective of the age assayed. Upon recombination and following a 2-week chase in 6-month-old mice, tdTomato-labeled satellite cells fused into myofibers as 20, 50, and 80 % of hindlimb, extra ocular, and diaphragm myofibers, respectively, were tdTomato+. Satellite cells contribute to uninjured myofibers either following a cell division or directly without an intervening cell division.
Conclusions: The frequency of satellite cell fusion into the skeletal muscle fibers is greater than previously estimated, suggesting an important functional role for satellite cell fusion into adult myofibers and a requirement for active maintenance of satellite cell numbers in uninjured skeletal muscle.
Keywords: Homeostasis; Pax7; Satellite cell; Skeletal muscle.
Figures



References
-
- Sousa-Victor P, García-Prat L, Serrano AL, Perdiguero E, Muñoz-Cánoves P. Muscle stem cell aging: regulation and rejuvenation. Trends Endocrinol Metab. 2015. doi:10.1016/j.tem.2015.03.006. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

