Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials
- PMID: 26669566
- PMCID: PMC4704538
- DOI: 10.1186/s13075-015-0880-2
Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials
Abstract
Background: Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA). Tofacitinib modulates the signaling of cytokines that are integral to lymphocyte activation, proliferation, and function. Thus, tofacitinib therapy may result in suppression of multiple elements of the immune response. Serious infections have been reported in tofacitinib RA trials. However, limited head-to-head comparator data were available within the tofacitinib RA development program to directly compare rates of serious infections with tofacitinib relative to biologic agents, and specifically adalimumab (employed as an active control agent in two randomized controlled trials of tofacitinib).
Methods: A systematic literature search of data from interventional randomized controlled trials and long-term extension studies with biologics in RA was carried out. Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) consensus was followed for reporting results of the review and meta-analysis. Incidence rates (unique patients with events/100 patient-years) for each therapy were estimated based on data from randomized controlled trials and long-term extension studies using a random-effects model. Relative and absolute risk comparisons versus placebo used Mantel-Haenszel methods.
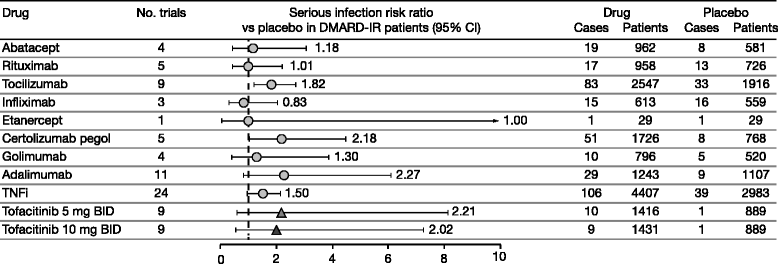
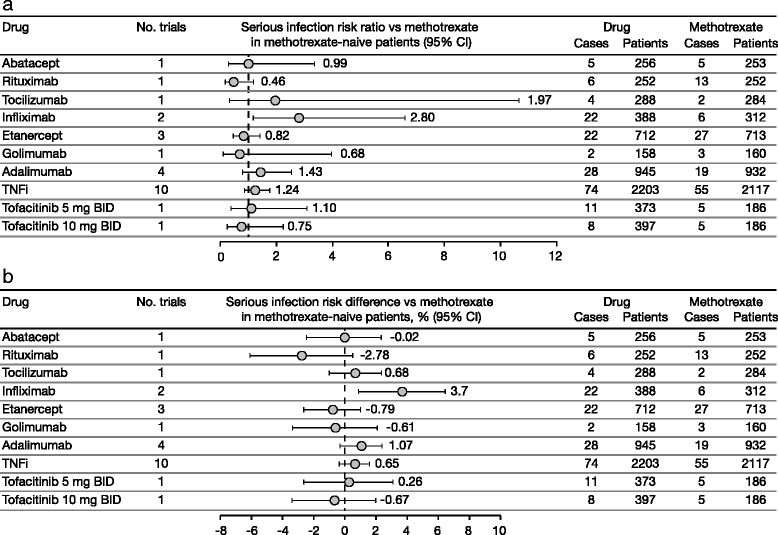
Results: The search produced 657 hits. In total, 66 randomized controlled trials and 22 long-term extension studies met the selection criteria. Estimated incidence rates (95% confidence intervals [CIs]) for abatacept, rituximab, tocilizumab, and tumor necrosis factor inhibitors were 3.04 (2.49, 3.72), 3.72 (2.99, 4.62), 5.45 (4.26, 6.96), and 4.90 (4.41, 5.44), respectively. Incidence rates (95% CIs) for tofacitinib 5 and 10 mg twice daily (BID) in phase 3 trials were 3.02 (2.25, 4.05) and 3.00 (2.24, 4.02), respectively. Corresponding incidence rates in long-term extension studies were 2.50 (2.05, 3.04) and 3.19 (2.74, 3.72). The risk ratios (95% CIs) versus placebo for tofacitinib 5 and 10 mg BID were 2.21 (0.60, 8.14) and 2.02 (0.56, 7.28), respectively. Risk differences (95% CIs) versus placebo for tofacitinib 5 and 10 mg BID were 0.38% (-0.24%, 0.99%) and 0.40% (-0.22%, 1.02%), respectively.
Conclusions: In interventional studies, the risk of serious infections with tofacitinib is comparable to published rates for biologic disease-modifying antirheumatic drugs in patients with moderate to severely active RA.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

