Reverse Engineering Applied to Red Human Hair Pheomelanin Reveals Redox-Buffering as a Pro-Oxidant Mechanism
- PMID: 26669666
- PMCID: PMC4680885
- DOI: 10.1038/srep18447
Reverse Engineering Applied to Red Human Hair Pheomelanin Reveals Redox-Buffering as a Pro-Oxidant Mechanism
Abstract
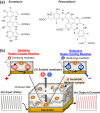
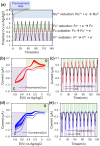
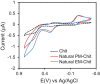
Pheomelanin has been implicated in the increased susceptibility to UV-induced melanoma for people with light skin and red hair. Recent studies identified a UV-independent pathway to melanoma carcinogenesis and implicated pheomelanin's pro-oxidant properties that act through the generation of reactive oxygen species and/or the depletion of cellular antioxidants. Here, we applied an electrochemically-based reverse engineering methodology to compare the redox properties of human hair pheomelanin with model synthetic pigments and natural eumelanin. This methodology exposes the insoluble melanin samples to complex potential (voltage) inputs and measures output response characteristics to assess redox activities. The results demonstrate that both eumelanin and pheomelanin are redox-active, they can rapidly (sec-min) and repeatedly redox-cycle between oxidized and reduced states, and pheomelanin possesses a more oxidative redox potential. This study suggests that pheomelanin's redox-based pro-oxidant activity may contribute to sustaining a chronic oxidative stress condition through a redox-buffering mechanism.
Figures






References
-
- Wakamatsu K., Ohtara K. & Ito S. Chemical analysis of late stages of pheomelanogenesis: conversion of dihydrobenzothiazine to a benzothiazole structure. Pigment Cell Melanoma Res 22, 474–486 (2009). - PubMed
-
- Napolitano A., Panzella L., Leone L. & D’Ischia M. Red Hair Benzothiazines and Benzothiazoles: Mutation-Inspired Chemistry in the Quest for Functionality. Acc. Chem. Res. 46, 519–528 (2013). - PubMed
-
- Miyamura Y. et al.. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 20, 2–13 (2007). - PubMed
-
- Olsen C. M., Carroll H. J. & Whiteman D. C. Estimating the attributable fraction for melanoma: a meta-analysis of pigmentary characteristics and freckling. Int. J. Cancer 127, 2430–2445 (2010). - PubMed
-
- Sarna T., Menon I. A. & Sealy R. C. Photosensitization of melanins: a comparative study. Photochem. Photobiol. 42, 529–532 (1985). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

