LUBAC-Recruited CYLD and A20 Regulate Gene Activation and Cell Death by Exerting Opposing Effects on Linear Ubiquitin in Signaling Complexes
- PMID: 26670046
- PMCID: PMC4688036
- DOI: 10.1016/j.celrep.2015.11.009
LUBAC-Recruited CYLD and A20 Regulate Gene Activation and Cell Death by Exerting Opposing Effects on Linear Ubiquitin in Signaling Complexes
Abstract
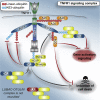
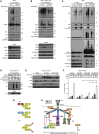
Ubiquitination and deubiquitination are crucial for assembly and disassembly of signaling complexes. LUBAC-generated linear (M1) ubiquitin is important for signaling via various immune receptors. We show here that the deubiquitinases CYLD and A20, but not OTULIN, are recruited to the TNFR1- and NOD2-associated signaling complexes (TNF-RSC and NOD2-SC), at which they cooperate to limit gene activation. Whereas CYLD recruitment depends on its interaction with LUBAC, but not on LUBAC's M1-chain-forming capacity, A20 recruitment requires this activity. Intriguingly, CYLD and A20 exert opposing effects on M1 chain stability in the TNF-RSC and NOD2-SC. While CYLD cleaves M1 chains, and thereby sensitizes cells to TNF-induced death, A20 binding to them prevents their removal and, consequently, inhibits cell death. Thus, CYLD and A20 cooperatively restrict gene activation and regulate cell death via their respective activities on M1 chains. Hence, the interplay between LUBAC, M1-ubiquitin, CYLD, and A20 is central for physiological signaling through innate immune receptors.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Bignell G.R., Warren W., Seal S., Takahashi M., Rapley E., Barfoot R., Green H., Brown C., Biggs P.J., Lakhani S.R. Identification of the familial cylindromatosis tumour-suppressor gene. Nat. Genet. 2000;25:160–165. - PubMed
-
- Bosanac I., Wertz I.E., Pan B., Yu C., Kusam S., Lam C., Phu L., Phung Q., Maurer B., Arnott D. Ubiquitin binding to A20 ZnF4 is required for modulation of NF-κB signaling. Mol. Cell. 2010;40:548–557. - PubMed
-
- Brummelkamp T.R., Nijman S.M., Dirac A.M., Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

