Intravenous Single-Dose Toxicity of Redaporfin-Based Photodynamic Therapy in Rodents
- PMID: 26670231
- PMCID: PMC4691106
- DOI: 10.3390/ijms161226162
Intravenous Single-Dose Toxicity of Redaporfin-Based Photodynamic Therapy in Rodents
Abstract
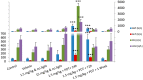
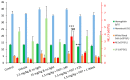
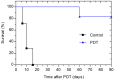
We assessed the tolerability and safety in rodents of a single intravenous (i.v.) dose of redaporfin, a novel photosensitizer for Photodynamic Therapy (PDT) of cancer. Two approaches were used to evaluate acute toxicity: (i) a dose escalation study in BALB/c mice to evaluate the maximum tolerated dose of redaporfin; and (ii) a safety toxicology study in Wistar rats, of a single dose of redaporfin, with or without illumination, to evaluate possible signs of systemic toxicity. Redaporfin formulation was well tolerated by mice, with no signs of adverse reactions up to 75 mg/kg. In rats, there were no relevant changes, except for a significant, but transient, increase in the blood serum markers for hepatic function and muscle integrity, and also on neutrophil counts, observed after the application of light. The overall results showed that redaporfin-PDT is very well tolerated. No abnormalities were observed, including reactions at the injection site or skin phototoxicity, although the animals were maintained in normal indoor lighting. Redaporfin also showed a high efficacy in the treatment of male BALB/c mice with subcutaneously implanted colon (CT26) tumours. Vascular-PDT with 1.5 mg/kg redaporfin and a light dose of 74 J/cm² led to the complete tumour regression in 83% of the mice.
Keywords: bacteriochlorin; cancer treatment; intravenous formulation; photodynamic therapy; redaporfin; rodents; single-dose toxicity.
Figures
References
-
- ICH, Guideline S9—Nonclinical Evaluation for Anticancer Pharmaceuticals. [(accessed on 29 October 2015)]. Available online: http://122.3.253.116/attachments/article/99526/S9%20Step%204.pdf. URL.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

