Integrative Network Analysis Combined with Quantitative Phosphoproteomics Reveals Transforming Growth Factor-beta Receptor type-2 (TGFBR2) as a Novel Regulator of Glioblastoma Stem Cell Properties
- PMID: 26670566
- PMCID: PMC4813685
- DOI: 10.1074/mcp.M115.049999
Integrative Network Analysis Combined with Quantitative Phosphoproteomics Reveals Transforming Growth Factor-beta Receptor type-2 (TGFBR2) as a Novel Regulator of Glioblastoma Stem Cell Properties
Abstract
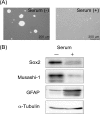
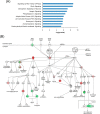
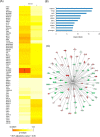
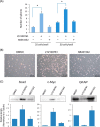
Glioblastoma is one of the most malignant brain tumors with poor prognosis and their development and progression are known to be driven by glioblastoma stem cells. Although glioblastoma stem cells lose their cancer stem cell properties during cultivation in serum-containing medium, little is known about the molecular mechanisms regulating signaling alteration in relation to reduction of stem cell-like characteristics. To elucidate the global phosphorylation-related signaling events, we performed a SILAC-based quantitative phosphoproteome analysis of serum-induced dynamics in glioblastoma stem cells established from the tumor tissues of the patient. Among a total of 2876 phosphorylation sites on 1584 proteins identified in our analysis, 732 phosphorylation sites on 419 proteins were regulated through the alteration of stem cell-like characteristics. The integrative computational analyses based on the quantified phosphoproteome data revealed the relevant changes of phosphorylation levels regarding the proteins associated with cytoskeleton reorganization such as Rho family GTPase and Intermediate filament signaling, in addition to transforming growth factor-β receptor type-2 (TGFBR2) as a prominent upstream regulator involved in the serum-induced phosphoproteome regulation. The functional association of transforming growth factor-β receptor type-2 with stem cell-like properties was experimentally validated through signaling perturbation using the corresponding inhibitors, which indicated that transforming growth factor-β receptor type-2 could play an important role as a novel cell fate determinant in glioblastoma stem cell regulation.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Wen P. Y., and Kesari S. (2008) Malignant gliomas in adults. N. Engl. J. Med. 359, 492–507 - PubMed
-
- Singh S. K., Clarke I. D., Terasaki M., Bonn V. E., Hawkins C., Squire J., and Dirks P. B. (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res. 63, 5821–5828 - PubMed
-
- Singh S. K., Hawkins C., Clarke I. D., Squire J. A., Bayani J., Hide T., Henkelman R. M., Cusimano M. D., and Dirks P. B. (2004) Identification of human brain tumor initiating cells. Nature 432, 396–401 - PubMed
-
- Lee J., Kotliarova S., Kotliarov Y., Li A., Su Q., Donin N. M., Pastorino S., Purow B. W., Christopher N., Zhang W., Park J. K., and Fine H. A. (2006) Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9, 391–403 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

