Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial
- PMID: 26670617
- PMCID: PMC5724392
- DOI: 10.1016/S0140-6736(15)01041-7
Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial
Abstract
Background: For children with sickle cell anaemia and high transcranial doppler (TCD) flow velocities, regular blood transfusions can effectively prevent primary stroke, but must be continued indefinitely. The efficacy of hydroxycarbamide (hydroxyurea) in this setting is unknown; we performed the TWiTCH trial to compare hydroxyurea with standard transfusions.
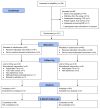
Methods: TWiTCH was a multicentre, phase 3, randomised, open-label, non-inferiority trial done at 26 paediatric hospitals and health centres in the USA and Canada. We enrolled children with sickle cell anaemia who were aged 4-16 years and had abnormal TCD flow velocities (≥ 200 cm/s) but no severe vasculopathy. After screening, eligible participants were randomly assigned 1:1 to continue standard transfusions (standard group) or hydroxycarbamide (alternative group). Randomisation was done at a central site, stratified by site with a block size of four, and an adaptive randomisation scheme was used to balance the covariates of baseline age and TCD velocity. The study was open-label, but TCD examinations were read centrally by observers masked to treatment assignment and previous TCD results. Participants assigned to standard treatment continued to receive monthly transfusions to maintain 30% sickle haemoglobin or lower, while those assigned to the alternative treatment started oral hydroxycarbamide at 20 mg/kg per day, which was escalated to each participant's maximum tolerated dose. The treatment period lasted 24 months from randomisation. The primary study endpoint was the 24 month TCD velocity calculated from a general linear mixed model, with the non-inferiority margin set at 15 cm/s. The primary analysis was done in the intention-to-treat population and safety was assessed in all patients who received at least one dose of assigned treatment. This study is registered with ClinicalTrials.gov, number NCT01425307.
Findings: Between Sept 20, 2011, and April 17, 2013, 159 patients consented and enrolled in TWiTCH. 121 participants passed screening and were then randomly assigned to treatment (61 to transfusions and 60 to hydroxycarbamide). At the first scheduled interim analysis, non-inferiority was shown and the sponsor terminated the study. Final model-based TCD velocities were 143 cm/s (95% CI 140-146) in children who received standard transfusions and 138 cm/s (135-142) in those who received hydroxycarbamide, with a difference of 4·54 (0·10-8·98). Non-inferiority (p=8·82 × 10(-16)) and post-hoc superiority (p=0·023) were met. Of 29 new neurological events adjudicated centrally by masked reviewers, no strokes were identified, but three transient ischaemic attacks occurred in each group. Magnetic resonance brain imaging and angiography (MRI and MRA) at exit showed no new cerebral infarcts in either treatment group, but worsened vasculopathy in one participant who received standard transfusions. 23 severe adverse events in nine (15%) patients were reported for hydroxycarbamide and ten serious adverse events in six (10%) patients were reported for standard transfusions. The most common serious adverse event in both groups was vaso-occlusive pain (11 events in five [8%] patients with hydroxycarbamide and three events in one [2%] patient for transfusions).
Interpretation: For high-risk children with sickle cell anaemia and abnormal TCD velocities who have received at least 1 year of transfusions, and have no MRA-defined severe vasculopathy, hydroxycarbamide treatment can substitute for chronic transfusions to maintain TCD velocities and help to prevent primary stroke.
Funding: National Heart, Lung, and Blood Institute, National Institutes of Health.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
Hydroxyurea is not approved by the US FDA for use in children with sickle cell anemia, and the TWiTCH trial was performed under FDA IND #67289 with cross-reference to FDA IND #111926. Dr. Ware is a consultant for Bayer Pharmaceuticals and Global Blood Therapeutics; receives research support from Bristol Myers-Squibb, Addmedica, and Biomedomics Inc.; and serves on a Data and Safety Monitoring Board for Eli Lilly. Dr. Odame serves as a consultant to Novartis, and sits on an Advisory Board to ApoPharma and Global Blood Therapeutics. Dr. Owen serves on the Speaker’s Bureau of Novartis. Dr. Rogers is a consultant to ApoPharma and on the Speaker’s Bureau for Bio-Rad Labs. Dr. Kwiatkowski is a consultant for Shire and Sideris, and receives research funding from ApoPharma. Dr. Heeney serves on the Scientific Advisory Board of Sancilio and Company. Dr. Imran is on the Speaker’s Bureau of NovoNordisk. Dr. Nottage is now employed by Janssen Pharmaceuticals, Inc. Dr. Wood is a consultant to ApoPharma, Biomed Informations, ISIS Pharmaceuticals, Celgene, AMAG, and Pfizer; receives research support from AMAG and Philips Healthcare; and serves as a Medical Advisor for ApoPharma. Dr. Cohen is a consultant to Novartis and serves on a Data and Safety Monitoring Board for an ApoPharma-sponsored clinical trial. None of these disclosures is relevant to the results and conclusions of the TWiTCH trial.
Nothing to disclose: BRD, WHS, RCB, BA, SS, BF, AG, LLJ, LH, CG, CP, MTL, SJ, STM, CR, TAK, SN, OA, MR, AAT, JAR, KJH, DR, JC, MJB, AK, NP, LP, PW, JL, NAM, SES, NLCL, SP, RJA
Figures



Comment in
-
New option for primary stroke prevention in sickle cell anaemia.Lancet. 2016 Feb 13;387(10019):626-627. doi: 10.1016/S0140-6736(15)01130-7. Epub 2015 Dec 6. Lancet. 2016. PMID: 26670618 No abstract available.
-
Treating sickle cell anaemia: the TWiTCH trial.Lancet. 2016 Sep 3;388(10048):960-1. doi: 10.1016/S0140-6736(16)31493-3. Epub 2016 Sep 1. Lancet. 2016. PMID: 27598673 No abstract available.
-
Treating sickle cell anaemia: the TWiTCH trial.Lancet. 2016 Sep 3;388(10048):960. doi: 10.1016/S0140-6736(16)31492-1. Epub 2016 Sep 1. Lancet. 2016. PMID: 27598674 No abstract available.
References
-
- Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: Rates and risk factors. Blood. 1998;91:288–94. - PubMed
-
- Adams R, McKie V, Nichols F, et al. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326:605–10. - PubMed
-
- Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusion in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. - PubMed
-
- Adams RJ for the STOP 2 Trial Investigators. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005;353:2769–78. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

