Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors
- PMID: 26670632
- PMCID: PMC4760094
- DOI: 10.1182/blood-2015-09-671834
Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors
Abstract
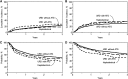
We evaluated 917 adult lymphoma patients who received haploidentical (n = 185) or HLA-matched unrelated donor (URD) transplantation either with (n = 241) or without antithymocyte globulin (ATG; n = 491) following reduced-intensity conditioning regimens. Haploidentical recipients received posttransplant cyclophosphamide-based graft-versus-host disease (GVHD) prophylaxis, whereas URD recipients received calcineurin inhibitor-based prophylaxis. Median follow-up of survivors was 3 years. The 100-day cumulative incidence of grade III-IV acute GVHD on univariate analysis was 8%, 12%, and 17% in the haploidentical, URD without ATG, and URD with ATG groups, respectively (P = .44). Corresponding 1-year rates of chronic GVHD on univariate analysis were 13%, 51%, and 33%, respectively (P < .001). On multivariate analysis, grade III-IV acute GVHD was higher in URD without ATG (P = .001), as well as URD with ATG (P = .01), relative to haploidentical transplants. Similarly, relative to haploidentical transplants, risk of chronic GVHD was higher in URD without ATG and URD with ATG (P < .0001). Cumulative incidence of relapse/progression at 3 years was 36%, 28%, and 36% in the haploidentical, URD without ATG, and URD with ATG groups, respectively (P = .07). Corresponding 3-year overall survival (OS) was 60%, 62%, and 50% in the 3 groups, respectively, with multivariate analysis showing no survival difference between URD without ATG (P = .21) or URD with ATG (P = .16), relative to haploidentical transplants. Multivariate analysis showed no difference between the 3 groups in terms of nonrelapse mortality (NRM), relapse/progression, and progression-free survival (PFS). These data suggest that reduced-intensity conditioning haploidentical transplantation with posttransplant cyclophosphamide does not compromise early survival outcomes compared with matched URD transplantation, and is associated with significantly reduced risk of chronic GVHD.
© 2016 by The American Society of Hematology.
Figures

Comment in
-
Might haplo "be the (better) match"?Blood. 2016 Feb 18;127(7):799-800. doi: 10.1182/blood-2016-01-689042. Blood. 2016. PMID: 26893397 Free PMC article.
References
-
- Appelbaum FR. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia when a matched related donor is not available. Hematology Am Soc Hematol Educ Program. 2008;2008(1):412–417. - PubMed
-
- Henslee-Downey PJ, Parrish RS, MacDonald JS, et al. Combined in vitro and in vivo T lymphocyte depletion for the control of graft-versus-host disease following haploidentical marrow transplant. Transplantation. 1996;61(5):738–745. - PubMed
-
- Kanda Y, Oshima K, Asano-Mori Y, et al. In vivo alemtuzumab enables haploidentical human leukocyte antigen-mismatched hematopoietic stem-cell transplantation without ex vivo graft manipulation. Transplantation. 2005;79(10):1351–1357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

