Autophagy postpones apoptotic cell death in PRRSV infection through Bad-Beclin1 interaction
- PMID: 26670824
- PMCID: PMC4994821
- DOI: 10.1080/21505594.2015.1131381
Autophagy postpones apoptotic cell death in PRRSV infection through Bad-Beclin1 interaction
Abstract
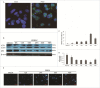
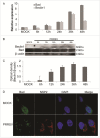
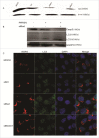
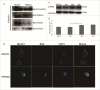
Autophagy and apoptosis play significant roles in PRRSV infection and replication. However, the interaction between these 2 processes in PRRSV replication is still far from been completely understood. In our studies, the exposure of MARC-145 cells to PRRSV confirmed the activation of autophagy and subsequent induction of apoptosis. The inhibition of autophagy by 3-methyladenine (3-MA) caused a significant increase in PRRSV-induced apoptosis, showing a potential connection between both mechanisms. Moreover, we observed an increase in Bad expression (a pro-apoptotic protein) and Beclin1 (an autophagy regulator) in virus-infected cells up to 36h. Co-immunoprecipitation assays showed the formation of Bad and Beclin1 complex in PRRSV infected cells. Accordingly, Bad co-localized with Beclin1 in MARC-145 infected cells. Knockdown of Beclin1 significantly decreased PRRSV replication and PRRSV-induced autophagy, while Bad silencing resulted in increased autophagy and enhanced viral replication. Furthermore, PRRSV infection phosphorylated Bad (Ser112) to promote cellular survival. These results demonstrate that autophagy can favor PRRSV replication by postponing apoptosis through the formation of a Bad-Beclin1 complex.
Keywords: Apoptosis; Autophagy; Bad; Beclin1; Porcine reproductive and respiratory syndrome virus.
Figures


References
-
- Thorburn A. Apoptosis and autophagy: regulatory connections between two supposedly different processes. Apoptosis 2008; 13:1-9; PMID:17990121; http://dx.doi.org/10.1007/s10495-007-0154-9 - DOI - PMC - PubMed
-
- Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, Stolz DB, Shao ZM, Yin XM. Differential effects of endoplasmic reticulum stress induced autophagy on cell survival. J Biol Chem 2007; 282(7):4702-10; PMID:17135238; http://dx.doi.org/10.1074/jbc.M609267200 - DOI - PubMed
-
- Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. BiochimBiophysActa 2013; 1833(12):3448-59 - PubMed
-
- Wang W, Zhou J, Shi J, Zhang Y, Liu S, Liu Y, Zheng D. Human T-cell leukemia virus type 1 Tax-deregulated autophagy pathway and c-FLIP expression contribute to resistance against death receptor-mediated apoptosis. J Virol 2014; 88(5):2786-98; PMID:24352466; http://dx.doi.org/10.1128/JVI.03025-13 - DOI - PMC - PubMed
-
- Gannagé M, Dormann D, Albrecht R, Dengjel J, Torossi T, Rämer PC, Lee M, Strowig T, Arrey F, Conenello G, et al. . Matrix protein 2 of influenza A virus blocks autophagosome fusion with lysosomes. Cell Host Microbe 2009; 6(4):367-80; http://dx.doi.org/10.1016/j.chom.2009.09.005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
