Sustained intraocular VEGF neutralization results in retinal neurodegeneration in the Ins2(Akita) diabetic mouse
- PMID: 26671074
- PMCID: PMC4680939
- DOI: 10.1038/srep18316
Sustained intraocular VEGF neutralization results in retinal neurodegeneration in the Ins2(Akita) diabetic mouse
Abstract
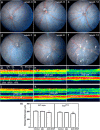
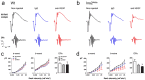
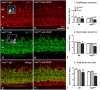
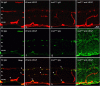
Current therapies that target vascular endothelial growth factor (VEGF) have become a mainstream therapy for the management of diabetic macular oedema. The treatment involves monthly repeated intravitreal injections of VEGF inhibitors. VEGF is an important growth factor for many retinal cells, including different types of neurons. In this study, we investigated the adverse effect of multiple intravitreal anti-VEGF injections (200 ng/μl/eye anti-mouse VEGF164, once every 2 weeks totalling 5-6 injections) to retinal neurons in Ins2(Akita) diabetic mice. Funduscopic examination revealed the development of cotton wool spot-like lesions in anti-VEGF treated Ins2(Akita) mice after 5 injections. Histological investigation showed focal swellings of retinal nerve fibres with neurofilament disruption. Furthermore, anti-VEGF-treated Ins2(Akita) mice exhibited impaired electroretinographic responses, characterized by reduced scotopic a- and b-wave and oscillatory potentials. Immunofluorescent staining revealed impairment of photoreceptors, disruptions of synaptic structures and loss of amacrine and retinal ganglion cells in anti-VEGF treated Ins2(Akita) mice. Anti-VEGF-treated WT mice also presented mild amacrine and ganglion cell death, but no overt abnormalities in photoreceptors and synaptic structures. At the vascular level, exacerbated albumin leakage was observed in anti-VEGF injected diabetic mice. Our results suggest that sustained intraocular VEGF neutralization induces retinal neurodegeneration and vascular damage in the diabetic eye.
Figures





References
-
- Klein R., Klein B. E., Moss S. E., Davis M. D. & DeMets D. L. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol 102, 520–526 (1984). - PubMed
-
- Klein B. E. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol 14, 179–183 (2007). - PubMed
-
- Klein R., Klein B. E., Moss S. E., Davis M. D. & DeMets D. L. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 102, 527–532 (1984). - PubMed
-
- Diabetes C. et al. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983-2005). Arch Intern Med 169, 1307–1316 (2009). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

