Unravelling the immunological roles of dipeptidyl peptidase 4 (DPP4) activity and/or structure homologue (DASH) proteins
- PMID: 26671446
- PMCID: PMC4872383
- DOI: 10.1111/cei.12757
Unravelling the immunological roles of dipeptidyl peptidase 4 (DPP4) activity and/or structure homologue (DASH) proteins
Abstract
Dipeptidyl peptidase (DPP) 4 (CD26, DPP4) is a multi-functional protein involved in T cell activation by co-stimulation via its association with adenosine deaminase (ADA), caveolin-1, CARMA-1, CD45, mannose-6-phosphate/insulin growth factor-II receptor (M6P/IGFII-R) and C-X-C motif receptor 4 (CXC-R4). The proline-specific dipeptidyl peptidase also modulates the bioactivity of several chemokines. However, a number of enzymes displaying either DPP4-like activities or representing structural homologues have been discovered in the past two decades and are referred to as DPP4 activity and/or structure homologue (DASH) proteins. Apart from DPP4, DASH proteins include fibroblast activation protein alpha (FAP), DPP8, DPP9, DPP4-like protein 1 (DPL1, DPP6, DPPX L, DPPX S), DPP4-like protein 2 (DPL2, DPP10) from the DPP4-gene family S9b and structurally unrelated enzyme DPP2, displaying DPP4-like activity. In contrast, DPP6 and DPP10 lack enzymatic DPP4-like activity. These DASH proteins play important roles in the immune system involving quiescence (DPP2), proliferation (DPP8/DPP9), antigen-presenting (DPP9), co-stimulation (DPP4), T cell activation (DPP4), signal transduction (DPP4, DPP8 and DPP9), differentiation (DPP4, DPP8) and tissue remodelling (DPP4, FAP). Thus, they are involved in many pathophysiological processes and have therefore been proposed for potential biomarkers or even drug targets in various cancers (DPP4 and FAP) and inflammatory diseases (DPP4, DPP8/DPP9). However, they also pose the challenge of drug selectivity concerning other DASH members for better efficacy and/or avoidance of unwanted side effects. Therefore, this review unravels the complex roles of DASH proteins in immunology.
Keywords: CD26; DPP4 activity and/or structure homologue proteins (DASH); antigen presentation/processing; co-stimulation; signal transduction.
© 2016 British Society for Immunology.
Figures
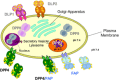
 , glycosylation.
, glycosylation.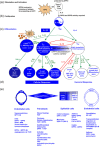
References
-
- Abbott CA, Yu DM, Woollatt E, Sutherland GR, McCaughan GW, Gorrell MD. Cloning, expression and chromosomal localization of a novel human dipeptidyl peptidase (DPP) IV homolog, DPP8. Eur J Biochem 2000; 267:6140–50. - PubMed
-
- Olsen C, Wagtmann N. Identification and characterization of human DPP9, a novel homologue of dipeptidyl peptidase IV. Gene 2002; 299:185–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

