Zebrafish heart as a model for human cardiac electrophysiology
- PMID: 26671745
- PMCID: PMC4960994
- DOI: 10.1080/19336950.2015.1121335
Zebrafish heart as a model for human cardiac electrophysiology
Abstract
The zebrafish (Danio rerio) has become a popular model for human cardiac diseases and pharmacology including cardiac arrhythmias and its electrophysiological basis. Notably, the phenotype of zebrafish cardiac action potential is similar to the human cardiac action potential in that both have a long plateau phase. Also the major inward and outward current systems are qualitatively similar in zebrafish and human hearts. However, there are also significant differences in ionic current composition between human and zebrafish hearts, and the molecular basis and pharmacological properties of human and zebrafish cardiac ionic currents differ in several ways. Cardiac ionic currents may be produced by non-orthologous genes in zebrafish and humans, and paralogous gene products of some ion channels are expressed in the zebrafish heart. More research on molecular basis of cardiac ion channels, and regulation and drug sensitivity of the cardiac ionic currents are needed to enable rational use of the zebrafish heart as an electrophysiological model for the human heart.
Keywords: calcium channels; cardiac action potential; cardiac ionic currents; potassium channels; sodium channels.
Figures
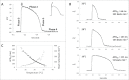

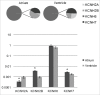
References
-
- Abi-Gerges N, Holkham H, Jones EM, Pollard CE, Valentin JP, Robertson GA. hERG subunit composition determines differential drug sensitivity. Br J Pharmacol 2011; 164:419–32; PMID:21449979; http://dx.doi.org/10.1111/j.1476-5381.2011.01378.x - DOI - PMC - PubMed
-
- Alday A, Alonso H, Gallego M, Urrutia J, Letamendia A, Callol C, Casis O. Ionic channels underlying the ventricular action potential in zebrafish embryo. Pharmacol Res 2014; 84:26–31; PMID:24747832; http://dx.doi.org/10.1016/j.phrs.2014.03.011 - DOI - PubMed
-
- Arnaout R, Ferrer T, Huisken J, Spitzer K, Stainier DY, Tristani-Firouzi M, Chi NC. Zebrafish model for human long QT syndrome. Proc Natl Acad Sci U S A 2007; 104:11316–21; PMID:17592134; http://dx.doi.org/10.1073/pnas.0702724104 - DOI - PMC - PubMed
-
- Arrenberg AB, Stainier DY, Baier H, Huisken J. Optogenetic control of cardiac function. Science 2010; 330:971–4; PMID:21071670; http://dx.doi.org/10.1126/science.1195929 - DOI - PubMed
-
- Baker K, Warren KS, Yellen G, Fishman MC. Defective “pacemaker” current (Ih) in a zebrafish mutant with a slow heart rate. Proc Natl Acad Sci U S A 1997; 94:4554–9; http://dx.doi.org/10.1073/pnas.94.9.4554 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
