Decoding the stem cell quiescence cycle--lessons from yeast for regenerative biology
- PMID: 26672015
- PMCID: PMC5695657
- DOI: 10.1242/jcs.177758
Decoding the stem cell quiescence cycle--lessons from yeast for regenerative biology
Abstract
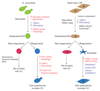
In the past decade, major advances have occurred in the understanding of mammalian stem cell biology, but roadblocks (including gaps in our fundamental understanding) remain in translating this knowledge to regenerative medicine. Interestingly, a close analysis of the Saccharomyces cerevisiae literature leads to an appreciation of how much yeast biology has contributed to the conceptual framework underpinning our understanding of stem cell behavior, to the point where such insights have been internalized into the realm of the known. This Opinion article focuses on one such example, the quiescent adult mammalian stem cell, and examines concepts underlying our understanding of quiescence that can be attributed to studies in yeast. We discuss the metabolic, signaling and gene regulatory events that control entry and exit into quiescence in yeast. These processes and events retain remarkable conservation and conceptual parallels in mammalian systems, and collectively suggest a regulated program beyond the cessation of cell division. We argue that studies in yeast will continue to not only reveal fundamental concepts in quiescence, but also leaven progress in regenerative medicine.
Keywords: Adult stem cell; Metabolism; Quiescence; Regeneration; Signaling pathways; Yeast.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures

References
-
- Becker AJ, McCulloch CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

