Pipecolic Acid Orchestrates Plant Systemic Acquired Resistance and Defense Priming via Salicylic Acid-Dependent and -Independent Pathways
- PMID: 26672068
- PMCID: PMC4746677
- DOI: 10.1105/tpc.15.00496
Pipecolic Acid Orchestrates Plant Systemic Acquired Resistance and Defense Priming via Salicylic Acid-Dependent and -Independent Pathways
Abstract
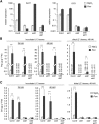
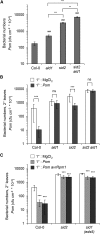
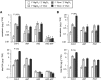
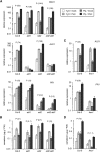
We investigated the relationships of the two immune-regulatory plant metabolites, salicylic acid (SA) and pipecolic acid (Pip), in the establishment of plant systemic acquired resistance (SAR), SAR-associated defense priming, and basal immunity. Using SA-deficient sid2, Pip-deficient ald1, and sid2 ald1 plants deficient in both SA and Pip, we show that SA and Pip act both independently from each other and synergistically in Arabidopsis thaliana basal immunity to Pseudomonas syringae. Transcriptome analyses reveal that SAR establishment in Arabidopsis is characterized by a strong transcriptional response systemically induced in the foliage that prepares plants for future pathogen attack by preactivating multiple stages of defense signaling and that SA accumulation upon SAR activation leads to the downregulation of photosynthesis and attenuated jasmonate responses systemically within the plant. Whereas systemic Pip elevations are indispensable for SAR and necessary for virtually the whole transcriptional SAR response, a moderate but significant SA-independent component of SAR activation and SAR gene expression is revealed. During SAR, Pip orchestrates SA-dependent and SA-independent priming of pathogen responses in a FLAVIN-DEPENDENT-MONOOXYGENASE1 (FMO1)-dependent manner. We conclude that a Pip/FMO1 signaling module acts as an indispensable switch for the activation of SAR and associated defense priming events and that SA amplifies Pip-triggered responses to different degrees in the distal tissue of SAR-activated plants.
© 2016 American Society of Plant Biologists. All rights reserved.
Figures





Similar articles
-
The Arabidopsis flavin-dependent monooxygenase FMO1 is an essential component of biologically induced systemic acquired resistance.Plant Physiol. 2006 Aug;141(4):1666-75. doi: 10.1104/pp.106.081257. Epub 2006 Jun 15. Plant Physiol. 2006. PMID: 16778014 Free PMC article.
-
N-hydroxy-pipecolic acid is a mobile metabolite that induces systemic disease resistance in Arabidopsis.Proc Natl Acad Sci U S A. 2018 May 22;115(21):E4920-E4929. doi: 10.1073/pnas.1805291115. Epub 2018 May 7. Proc Natl Acad Sci U S A. 2018. PMID: 29735713 Free PMC article.
-
Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity.Plant Cell. 2012 Dec;24(12):5123-41. doi: 10.1105/tpc.112.103564. Epub 2012 Dec 7. Plant Cell. 2012. PMID: 23221596 Free PMC article.
-
l-lysine metabolism to N-hydroxypipecolic acid: an integral immune-activating pathway in plants.Plant J. 2018 Oct;96(1):5-21. doi: 10.1111/tpj.14037. Epub 2018 Sep 1. Plant J. 2018. PMID: 30035374 Review.
-
Pipecolic acid: A positive regulator of systemic acquired resistance and plant immunity.Biochim Biophys Acta Gen Subj. 2025 Jun;1869(7):130808. doi: 10.1016/j.bbagen.2025.130808. Epub 2025 Apr 17. Biochim Biophys Acta Gen Subj. 2025. PMID: 40252741 Review.
Cited by
-
Broad-spectrum resistance mechanism of serine protease Sp1 in Bacillus licheniformis W10 via dual comparative transcriptome analysis.Front Microbiol. 2022 Oct 4;13:974473. doi: 10.3389/fmicb.2022.974473. eCollection 2022. Front Microbiol. 2022. PMID: 36267189 Free PMC article.
-
Chemokine-like MDL proteins modulate flowering time and innate immunity in plants.J Biol Chem. 2021 Jan-Jun;296:100611. doi: 10.1016/j.jbc.2021.100611. Epub 2021 Mar 30. J Biol Chem. 2021. PMID: 33798552 Free PMC article.
-
Isolation of Open Chromatin Identifies Regulators of Systemic Acquired Resistance.Plant Physiol. 2019 Oct;181(2):817-833. doi: 10.1104/pp.19.00673. Epub 2019 Jul 23. Plant Physiol. 2019. PMID: 31337712 Free PMC article.
-
Glycerol-3-phosphate mediates rhizobia-induced systemic signaling in soybean.Nat Commun. 2019 Nov 22;10(1):5303. doi: 10.1038/s41467-019-13318-8. Nat Commun. 2019. PMID: 31757957 Free PMC article.
-
The Hidden Potential of High-Throughput RNA-Seq Re-Analysis, a Case Study for DHDPS, Key Enzyme of the Aspartate-Derived Lysine Biosynthesis Pathway and Its Role in Abiotic and Biotic Stress Responses in Soybean.Plants (Basel). 2022 Jul 1;11(13):1762. doi: 10.3390/plants11131762. Plants (Basel). 2022. PMID: 35807714 Free PMC article.
References
-
- Acharya B.R., Raina S., Maqbool S.B., Jagadeeswaran G., Mosher S.L., Appel H.M., Schultz J.C., Klessig D.F., Raina R. (2007). Overexpression of CRK13, an Arabidopsis cysteine-rich receptor-like kinase, results in enhanced resistance to Pseudomonas syringae. Plant J. 50: 488–499. - PubMed
-
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. - PubMed
-
- Bartsch M., Gobbato E., Bednarek P., Debey S., Schultze J.L., Bautor J., Parker J.E. (2006). Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell 18: 1038–1051. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

