Systemic Immunity Requires SnRK2.8-Mediated Nuclear Import of NPR1 in Arabidopsis
- PMID: 26672073
- PMCID: PMC4707448
- DOI: 10.1105/tpc.15.00371
Systemic Immunity Requires SnRK2.8-Mediated Nuclear Import of NPR1 in Arabidopsis
Abstract
In plants, necrotic lesions occur at the site of pathogen infection through the hypersensitive response, which is followed by induction of systemic acquired resistance (SAR) in distal tissues. Salicylic acid (SA) induces SAR by activating NONEXPRESSER OF PATHOGENESIS-RELATED GENES1 (NPR1) through an oligomer-to-monomer reaction. However, SA biosynthesis is elevated only slightly in distal tissues during SAR, implying that SA-mediated induction of SAR requires additional factors. Here, we demonstrated that SA-independent systemic signals induce a gene encoding SNF1-RELATED PROTEIN KINASE 2.8 (SnRK2.8), which phosphorylates NPR1 during SAR. The SnRK2.8-mediated phosphorylation of NPR1 is necessary for its nuclear import. Notably, although SnRK2.8 transcription and SnRK2.8 activation are independent of SA signaling, the SnRK2.8-mediated induction of SAR requires SA. Together with the SA-mediated monomerization of NPR1, these observations indicate that SA signals and SnRK2.8-mediated phosphorylation coordinately function to activate NPR1 via a dual-step process in developing systemic immunity in Arabidopsis thaliana.
© 2015 American Society of Plant Biologists. All rights reserved.
Figures
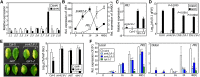
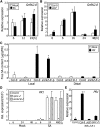



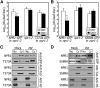
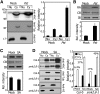
Comment in
-
Phosphorylation and Nuclear Localization of NPR1 in Systemic Acquired Resistance.Plant Cell. 2015 Dec;27(12):3291. doi: 10.1105/tpc.15.01020. Epub 2015 Dec 15. Plant Cell. 2015. PMID: 26672072 Free PMC article. No abstract available.
Similar articles
-
Abscisic acid promotes proteasome-mediated degradation of the transcription coactivator NPR1 in Arabidopsis thaliana.Plant J. 2016 Apr;86(1):20-34. doi: 10.1111/tpj.13141. Plant J. 2016. PMID: 26865090
-
Nuclear localization of NPR1 is required for regulation of salicylate tolerance, isochorismate synthase 1 expression and salicylate accumulation in Arabidopsis.J Plant Physiol. 2010 Jan 15;167(2):144-8. doi: 10.1016/j.jplph.2009.08.002. J Plant Physiol. 2010. PMID: 19716624
-
Strong suppression of systemic acquired resistance in Arabidopsis by NRR is dependent on its ability to interact with NPR1 and its putative repression domain.Mol Plant. 2008 May;1(3):552-9. doi: 10.1093/mp/ssn017. Epub 2008 Apr 22. Mol Plant. 2008. PMID: 19825560
-
NPR1 in JazzSet with Pathogen Effectors.Trends Plant Sci. 2018 Jun;23(6):469-472. doi: 10.1016/j.tplants.2018.04.007. Epub 2018 May 9. Trends Plant Sci. 2018. PMID: 29753632 Review.
-
Closing another gap in the plant SAR puzzle.Cell. 2003 Jun 27;113(7):815-6. doi: 10.1016/s0092-8674(03)00473-2. Cell. 2003. PMID: 12837238 Review.
Cited by
-
Plant-Microbe Interaction: Aboveground to Belowground, from the Good to the Bad.Int J Mol Sci. 2021 Sep 27;22(19):10388. doi: 10.3390/ijms221910388. Int J Mol Sci. 2021. PMID: 34638728 Free PMC article. Review.
-
Improvement of Verticillium Wilt Resistance by Applying Arbuscular Mycorrhizal Fungi to a Cotton Variety with High Symbiotic Efficiency under Field Conditions.Int J Mol Sci. 2018 Jan 13;19(1):241. doi: 10.3390/ijms19010241. Int J Mol Sci. 2018. PMID: 29342876 Free PMC article.
-
A self-balancing circuit centered on MoOsm1 kinase governs adaptive responses to host-derived ROS in Magnaporthe oryzae.Elife. 2020 Dec 4;9:e61605. doi: 10.7554/eLife.61605. Elife. 2020. PMID: 33275098 Free PMC article.
-
SnRK2s: Kinases or Substrates?Plants (Basel). 2025 Apr 9;14(8):1171. doi: 10.3390/plants14081171. Plants (Basel). 2025. PMID: 40284059 Free PMC article. Review.
-
Deciphering ABA/PYL gene family in flax: evolutionary analysis, and abiotic stress response.Plant Cell Rep. 2025 Jun 6;44(7):140. doi: 10.1007/s00299-025-03517-7. Plant Cell Rep. 2025. PMID: 40478340
References
-
- Berger S., Sinha A.K., Roitsch T. (2007). Plant physiology meets phytopathology: plant primary metabolism and plant-pathogen interactions. J. Exp. Bot. 58: 4019–4026. - PubMed
-
- Bolton M.D. (2009). Primary metabolism and plant defense--fuel for the fire. Mol. Plant Microbe Interact. 22: 487–497. - PubMed
-
- Boudsocq M., Barbier-Brygoo H., Laurière C. (2004). Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. J. Biol. Chem. 279: 41758–41766. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

