Efficacy and Safety of Vorapaxar in Non-ST-Segment Elevation Acute Coronary Syndrome Patients Undergoing Noncardiac Surgery
- PMID: 26672080
- PMCID: PMC4845287
- DOI: 10.1161/JAHA.115.002546
Efficacy and Safety of Vorapaxar in Non-ST-Segment Elevation Acute Coronary Syndrome Patients Undergoing Noncardiac Surgery
Abstract
Background: Perioperative antiplatelet agents potentially increase bleeding after non-ST-segment elevation (NSTE) acute coronary syndromes (ACS). The protease-activated receptor 1 antagonist vorapaxar reduced cardiovascular events and was associated with increased bleeding versus placebo in NSTE ACS, but its efficacy and safety in noncardiac surgery (NCS) remain unknown. We aimed to evaluate ischemic, bleeding, and long-term outcomes of vorapaxar in NCS after NSTE ACS.
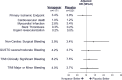
Methods and results: In the TRACER trial, 2202 (17.0%) patients underwent major or minor NCS after NSTE ACS over 1.5 years (median); continuing study treatment perioperatively was recommended. The primary ischemic end point for this analysis was cardiovascular death, myocardial infarction, stent thrombosis, or urgent revascularization within 30 days of NCS. Safety outcomes included 30-day NCS bleeding and GUSTO moderate/severe bleeding. Overall, 1171 vorapaxar and 1031 placebo patients underwent NCS. Preoperative aspirin and thienopyridine use was 96.8% versus 97.7% (P=0.235) and 89.1% versus 86.1% (P=0.036) for vorapaxar versus placebo, respectively. Within 30 days of NCS, no differences were observed in the primary ischemic end point between vorapaxar and placebo groups (3.4% versus 3.9%; adjusted odds ratio 0.81, 95% CI 0.50 to 1.33, P=0.41). Similarly, no differences in NCS bleeding (3.9% versus 3.4%; adjusted odds ratio 1.41, 95% CI 0.87 to 2.31, P=0.17) or GUSTO moderate/severe bleeding (4.2% versus 3.7%; adjusted odds ratio 1.15, 95% CI, 0.72 to 1.83, P=0.55) were observed. In a 30-day landmarked analysis, NCS patients had a higher long-term risk of the ischemic end point (adjusted hazard ratio 1.62, 95% CI 1.33 to 1.97, P<0.001) and GUSTO moderate/severe bleeding (adjusted hazard ratio 5.63, 95% CI 3.98 to 7.97, P<0.001) versus patients who did not undergo NCS, independent of study treatment.
Conclusion: NCS after NSTE ACS is common and associated with more ischemic outcomes and bleeding. Vorapaxar after NSTE ACS was not associated with increased perioperative ischemic or bleeding events in patients undergoing NCS.
Keywords: coronary disease; hemorrhage; noncardiac surgery; non–ST‐segment elevation acute coronary syndromes; surgery; vorapaxar.
© 2015 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures


Similar articles
-
Effect of age on efficacy and safety of vorapaxar in patients with non-ST-segment elevation acute coronary syndrome: Insights from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) trial.Am Heart J. 2016 Aug;178:176-84. doi: 10.1016/j.ahj.2016.05.012. Epub 2016 Jun 9. Am Heart J. 2016. PMID: 27502866
-
Use of thienopyridine prior to presentation with non-ST-segment elevation acute coronary syndrome and association with safety and efficacy of vorapaxar: insights from the TRACER trial.Eur Heart J Acute Cardiovasc Care. 2017 Mar;6(2):155-163. doi: 10.1177/2048872616633880. Epub 2016 Sep 20. Eur Heart J Acute Cardiovasc Care. 2017. PMID: 26895973 Clinical Trial.
-
Vorapaxar in patients with peripheral artery disease and acute coronary syndrome: insights from Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER).Am Heart J. 2014 Oct;168(4):588-96. doi: 10.1016/j.ahj.2014.06.017. Epub 2014 Jul 4. Am Heart J. 2014. PMID: 25262270 Clinical Trial.
-
Vorapaxar in the secondary prevention of atherothrombosis.Expert Rev Cardiovasc Ther. 2015 Dec;13(12):1293-305. doi: 10.1586/14779072.2015.1109447. Epub 2015 Nov 12. Expert Rev Cardiovasc Ther. 2015. PMID: 26559689 Review.
-
Vorapaxar in atherosclerotic disease management.Ann Pharmacother. 2015 May;49(5):599-606. doi: 10.1177/1060028015571410. Epub 2015 Feb 13. Ann Pharmacother. 2015. PMID: 25680760 Review.
References
-
- Hawn MT, Graham LA, Richman JS, Itani KF, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA. 2013;310:1462–1472. - PubMed
-
- To AC, Armstrong G, Zeng I, Webster MW. Noncardiac surgery and bleeding after percutaneous coronary intervention. Circ Cardiovasc Interv. 2009;2:213–221. - PubMed
-
- Wijeysundera DN, Wijeysundera HC, Yun L, Wąsowicz M, Beattie WS, Velianou JL, Ko DT. Risk of elective major noncardiac surgery after coronary stent insertion: a population‐based study. Circulation. 2012;126:1355–1362. - PubMed
-
- Goldman L, Caldera DL, Nussbaum SR, Southwick FS, Krogstad D, Murray B, Burke DS, O'Malley TA, Goroll AH, Caplan CH, Nolan J, Carabello B, Slater EE. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845–850. - PubMed
-
- Detsky AS, Abrams HB, McLaughlin JR, Drucker DJ, Sasson Z, Johnston N, Scott JG, Forbath N, Hilliard JR. Predicting cardiac complications in patients undergoing non‐cardiac surgery. J Gen Intern Med. 1986;1:211–219. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

