Prospective Validation of Pooled Prognostic Factors in Women with Advanced Cervical Cancer Treated with Chemotherapy with/without Bevacizumab: NRG Oncology/GOG Study
- PMID: 26672085
- PMCID: PMC4896296
- DOI: 10.1158/1078-0432.CCR-15-1346
Prospective Validation of Pooled Prognostic Factors in Women with Advanced Cervical Cancer Treated with Chemotherapy with/without Bevacizumab: NRG Oncology/GOG Study
Erratum in
-
Correction: Prospective Validation of Pooled Prognostic Factors in Women with Advanced Cervical Cancer Treated with Chemotherapy with/without Bevacizumab: NRG Oncology/GOG Study.Clin Cancer Res. 2016 Aug 1;22(15):3983. doi: 10.1158/1078-0432.CCR-16-1208. Clin Cancer Res. 2016. PMID: 27480871 No abstract available.
Abstract
Purpose: In the randomized phase III trial, Gynecologic Oncology Group (GOG) protocol 240, the incorporation of bevacizumab with chemotherapy significantly increased overall survival (OS) in women with advanced cervical cancer. A major objective of GOG-240 was to prospectively analyze previously identified pooled clinical prognostic factors known as the Moore criteria.
Experimental design: Potential negative factors included black race, performance status 1, pelvic disease, prior cisplatin, and progression-free interval <365 days. Risk categories included low-risk (0-1 factor), mid-risk (2-3 factors), and high-risk (4-5 factors). Each test of association was conducted at the 5% level of significance. Logistic regression and survival analysis was used to determine whether factors were prognostic or could be used to guide therapy.
Results: For the entire population (n = 452), high-risk patients had significantly worse OS (P < 0.0001). The HRs of death for treating with topotecan in low-risk, mid-risk, and high-risk subsets are 1.18 [95% confidence interval (CI), 0.63-2.24], 1.11 (95% CI, 0.82-1.5), and 0.84 (95% CI, 0.50-1.42), respectively. The HRs of death for treating with bevacizumab in low-risk, mid-risk, and high-risk subsets are 0.96 (95% CI, 0.51-1.83; P = 0.9087), 0.673 (95% CI, 0.5-0.91; P = 0.0094), and 0.536 (95% CI, 0.32-0.905; P = 0.0196), respectively.
Conclusions: This is the first prospectively validated scoring system in cervical cancer. The Moore criteria have real-world clinical applicability. Toxicity concerns may justify omission of bevacizumab in some low-risk patients where survival benefit is small. The benefit to receiving bevacizumab appears to be greatest in the moderate- and high-risk subgroups (5.8-month increase in median OS). Clin Cancer Res; 21(24); 5480-7. ©2015 AACR.
©2015 American Association for Cancer Research.
Figures


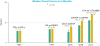
Similar articles
-
Bevacizumab for advanced cervical cancer: patient-reported outcomes of a randomised, phase 3 trial (NRG Oncology-Gynecologic Oncology Group protocol 240).Lancet Oncol. 2015 Mar;16(3):301-11. doi: 10.1016/S1470-2045(15)70004-5. Epub 2015 Jan 29. Lancet Oncol. 2015. PMID: 25638326 Free PMC article. Clinical Trial.
-
Improved survival with bevacizumab in advanced cervical cancer.N Engl J Med. 2014 Feb 20;370(8):734-43. doi: 10.1056/NEJMoa1309748. N Engl J Med. 2014. PMID: 24552320 Free PMC article. Clinical Trial.
-
The real-world efficacy and toxicity of first-line paclitaxel and cisplatin with bevacizumab in platinum-naïve primary stage IVB cervical cancer.Taiwan J Obstet Gynecol. 2025 Jan;64(1):61-67. doi: 10.1016/j.tjog.2020.07.035. Taiwan J Obstet Gynecol. 2025. PMID: 39794053
-
Chemotherapy for recurrent cervical cancer.Cancer Treat Rev. 2008 Nov;34(7):603-13. doi: 10.1016/j.ctrv.2008.05.006. Epub 2008 Jul 26. Cancer Treat Rev. 2008. PMID: 18657909 Review.
-
Chemotherapy for advanced, recurrent, and metastatic cervical cancer.J Natl Compr Canc Netw. 2008 Jan;6(1):53-7. doi: 10.6004/jnccn.2008.0006. J Natl Compr Canc Netw. 2008. PMID: 18267059 Review.
Cited by
-
Twenty-first century cervical cancer management: A historical perspective of the gynecologic oncology group/NRG oncology over the past twenty years.Gynecol Oncol. 2018 Sep;150(3):391-397. doi: 10.1016/j.ygyno.2018.06.023. Epub 2018 Jun 27. Gynecol Oncol. 2018. PMID: 29954593 Free PMC article. Review.
-
Increased genitourinary fistula rate after bevacizumab in recurrent cervical cancer patients initially treated with definitive radiochemotherapy and image-guided adaptive brachytherapy.Strahlenther Onkol. 2017 Dec;193(12):1056-1065. doi: 10.1007/s00066-017-1178-x. Epub 2017 Jul 18. Strahlenther Onkol. 2017. PMID: 28721510 Free PMC article.
-
Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240).Lancet. 2017 Oct 7;390(10103):1654-1663. doi: 10.1016/S0140-6736(17)31607-0. Epub 2017 Jul 27. Lancet. 2017. PMID: 28756902 Free PMC article. Clinical Trial.
-
Metronomic chemotherapy using oral cyclophosphamide and bevacizumab for recurrent cervical cancer: A multi-institutional retrospective study.Gynecol Oncol Rep. 2022 May 28;42:101013. doi: 10.1016/j.gore.2022.101013. eCollection 2022 Aug. Gynecol Oncol Rep. 2022. PMID: 36118995 Free PMC article.
-
The prognostic factors influencing overall survival in uterine cervical cancer with brain metastasis.Korean J Intern Med. 2019 Nov;34(6):1324-1332. doi: 10.3904/kjim.2018.051. Epub 2018 Oct 26. Korean J Intern Med. 2019. PMID: 30360020 Free PMC article.
References
-
- Tewari KS, Monk BJ. Invasive Cervical Cancer. In: DiSaia PJ, Creasman WT, editors. Clinical Gynecologic Oncology. 8th. Mosby; 2012.
-
- Monk BJ, Tewari KS, Koh WJ. Multi-modality therapy for locally advanced cervical carcinoma: State of the art and future directions. J Clin Oncol. 2007;25:2952–2965. - PubMed
-
- Tewari KS, Monk BJ. Gynecologic Oncology Group trials of chemotherapy for metastatic and recurrent cervical carcinoma. Curr Oncol Rep. 2005;7:419–434. - PubMed
-
- Tewari KS, Monk BJ. Beyond platinum for metastatic and recurrent carcinoma of the cervix. Onkologie. 2009;32:552–554. - PubMed

