Tendon development and musculoskeletal assembly: emerging roles for the extracellular matrix
- PMID: 26672092
- PMCID: PMC4689213
- DOI: 10.1242/dev.114777
Tendon development and musculoskeletal assembly: emerging roles for the extracellular matrix
Abstract
Tendons and ligaments are extracellular matrix (ECM)-rich structures that interconnect muscles and bones. Recent work has shown how tendon fibroblasts (tenocytes) interact with muscles via the ECM to establish connectivity and strengthen attachments under tension. Similarly, ECM-dependent interactions between tenocytes and cartilage/bone ensure that tendon-bone attachments form with the appropriate strength for the force required. Recent studies have also established a close lineal relationship between tenocytes and skeletal progenitors, highlighting the fact that defects in signals modulated by the ECM can alter the balance between these fates, as occurs in calcifying tendinopathies associated with aging. The dynamic fine-tuning of tendon ECM composition and assembly thus gives rise to the remarkable characteristics of this unique tissue type. Here, we provide an overview of the functions of the ECM in tendon formation and maturation that attempts to integrate findings from developmental genetics with those of matrix biology.
Keywords: Extracellular matrix; Ligament; Tendon; Tenocyte.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures
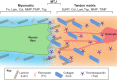

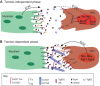
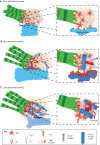
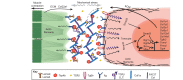
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

