Intrarenal renin-angiotensin system mediates fatty acid-induced ER stress in the kidney
- PMID: 26672616
- PMCID: PMC4971807
- DOI: 10.1152/ajprenal.00223.2015
Intrarenal renin-angiotensin system mediates fatty acid-induced ER stress in the kidney
Abstract
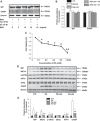
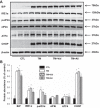
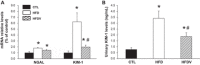
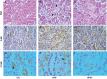
Obesity-related kidney disease is related to caloric excess promoting deleterious cellular responses. Accumulation of saturated free fatty acids in tubular cells produces lipotoxicity involving significant cellular dysfunction and injury. The objectives of this study were to elucidate the role of renin-angiotensin system (RAS) activation in saturated fatty acid-induced endoplasmic reticulum (ER) stress in cultured human proximal tubule epithelial cells (HK2) and in mice fed with a high-fat diet. Treatment with saturated fatty acid palmitic acid (PA; 0.8 mM) for 24 h induced ER stress in HK2, leading to an unfolded protein response as reflected by increased expressions of the ER chaperone binding immunoglobulin protein (BiP) and proapoptotic transcription factor C/EBP homologous protein (CHOP) protein as evaluated by immunoblotting. PA treatment also induced increased protein expression of inositol requiring protein 1α (IRE1α), phosphorylated eukaryotic initiation factor-α (eIF2α), and activating transcription factor 4 (ATF4) as well as activation of caspase-3. PA treatment was associated with increased angiotensin II levels in cultured medium. The angiotensin II type 1 receptor (AT1R) blocker valsartan or renin inhibitor aliskiren dramatically suppressed PA-induced upregulation of BiP, CHOP, IRE1α, p-eIF2α, and ATF4 in HK2 cells. In contrast, valsartan or aliskiren did not prevent ER stress induced by tunicamycin. C57BL/6 mice fed with a high-fat diet for 14 wk exhibited increased protein expressions of BiP and CHOP compared with control mice, which were significantly attenuated by the valsartan treatment. Increased angiotensin II levels in serum and urine were observed in mice fed with a high-fat diet when compared with controls. It is suggested that the intrarenal RAS activation may play an important role in diabetic kidney injury via mediating ER stress induced by saturated fatty acid.
Keywords: angiotensin II; kidney; saturated fatty acid; valsartan.
Copyright © 2016 the American Physiological Society.
Figures




Similar articles
-
Combination of Chymostatin and Aliskiren attenuates ER stress induced by lipid overload in kidney tubular cells.Lipids Health Dis. 2018 Jul 31;17(1):183. doi: 10.1186/s12944-018-0818-1. Lipids Health Dis. 2018. PMID: 30064425 Free PMC article.
-
Protective Effects of Glucagon-Like Peptide-1 Analog on Renal Tubular Injury in Mice on High-Fat Diet.Cell Physiol Biochem. 2017;41(3):1113-1124. doi: 10.1159/000464118. Epub 2017 Feb 28. Cell Physiol Biochem. 2017. PMID: 28245463
-
Cannabinoid receptor 1 mediates palmitic acid-induced apoptosis via endoplasmic reticulum stress in human renal proximal tubular cells.J Cell Physiol. 2010 Nov;225(3):654-63. doi: 10.1002/jcp.22255. J Cell Physiol. 2010. PMID: 20506110
-
Systemic effects of AGEs in ER stress induction in vivo.Glycoconj J. 2016 Aug;33(4):537-44. doi: 10.1007/s10719-016-9680-4. Epub 2016 May 28. Glycoconj J. 2016. PMID: 27236787 Review.
-
The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection.Front Immunol. 2019 Jan 4;9:3083. doi: 10.3389/fimmu.2018.03083. eCollection 2018. Front Immunol. 2019. PMID: 30662442 Free PMC article. Review.
Cited by
-
Nifedipine Exacerbates Lipogenesis in the Kidney via KIM-1, CD36, and SREBP Upregulation: Implications from an Animal Model for Human Study.Int J Mol Sci. 2020 Jun 19;21(12):4359. doi: 10.3390/ijms21124359. Int J Mol Sci. 2020. PMID: 32575412 Free PMC article.
-
Nonlinear association between atherogenic index of plasma and chronic kidney disease: a nationwide cross-sectional study.Lipids Health Dis. 2024 Sep 27;23(1):312. doi: 10.1186/s12944-024-02288-6. Lipids Health Dis. 2024. PMID: 39334373 Free PMC article.
-
Developmental Programming of Renal Function and Re-Programming Approaches.Front Pediatr. 2018 Feb 27;6:36. doi: 10.3389/fped.2018.00036. eCollection 2018. Front Pediatr. 2018. PMID: 29535992 Free PMC article. Review.
-
Adipose triglyceride lipase protects renal cell endocytosis in a Drosophila dietary model of chronic kidney disease.PLoS Biol. 2021 May 4;19(5):e3001230. doi: 10.1371/journal.pbio.3001230. eCollection 2021 May. PLoS Biol. 2021. PMID: 33945525 Free PMC article.
-
Serum triglycerides level is independently associated with renal outcomes in patients with non-dialysis chronic kidney disease: Results from KNOW-CKD study.Front Nutr. 2022 Nov 24;9:1037618. doi: 10.3389/fnut.2022.1037618. eCollection 2022. Front Nutr. 2022. PMID: 36505239 Free PMC article.
References
-
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2: 326–332, 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

