Quinomycin A targets Notch signaling pathway in pancreatic cancer stem cells
- PMID: 26673007
- PMCID: PMC4823101
- DOI: 10.18632/oncotarget.6560
Quinomycin A targets Notch signaling pathway in pancreatic cancer stem cells
Abstract
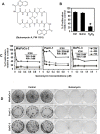
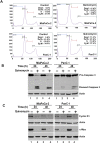
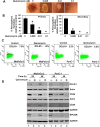
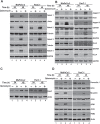
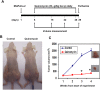
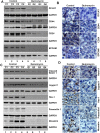
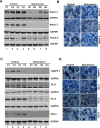
Cancer stem cells (CSCs) appear to explain many aspects of the neoplastic evolution of tumors and likely account for enhanced therapeutic resistance following treatment. Dysregulated Notch signaling, which affects CSCs plays an important role in pancreatic cancer progression. We have determined the ability of Quinomycin to inhibit CSCs and the Notch signaling pathway. Quinomycin treatment resulted in significant inhibition of proliferation and colony formation in pancreatic cancer cell lines, but not in normal pancreatic epithelial cells. Moreover, Quinomycin affected pancreatosphere formation. The compound also decreased the expression of CSC marker proteins DCLK1, CD44, CD24 and EPCAM. In addition, flow cytometry studies demonstrated that Quinomycin reduced the number of DCLK1+ cells. Furthermore, levels of Notch 1-4 receptors, their ligands Jagged1, Jagged2, DLL1, DLL3, DLL4 and the downstream target protein Hes-1 were reduced. The γ-secretase complex proteins, Presenilin 1, Nicastrin, Pen2, and APH-1, required for Notch activation also exhibited decreased expression. Ectopic expression of the Notch Intracellular Domain (NICD) partially rescued the cells from Quinomycin mediated growth suppression. To determine the effect of Quinomycin on tumor growth in vivo, nude mice carrying tumor xenografts were administered Quinomycin intraperitoneally every day for 21 days. Treatment with the compound significantly inhibited tumor xenograft growth, coupled with significant reduction in the expression of CSC markers and Notch signaling proteins. Together, these data suggest that Quinomycin is a potent inhibitor of pancreatic cancer that targets the stem cells by inhibiting Notch signaling proteins.
Keywords: DCLK1; Hes1; NICD; apoptosis; tumor xenograft.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Figures

Similar articles
-
Gamma-Secretase Inhibitor IX (GSI) Impairs Concomitant Activation of Notch and Wnt-Beta-Catenin Pathways in CD44+ Gastric Cancer Stem Cells.Stem Cells Transl Med. 2017 Mar;6(3):819-829. doi: 10.1002/sctm.16-0335. Epub 2017 Feb 3. Stem Cells Transl Med. 2017. PMID: 28186678 Free PMC article.
-
3,5-bis(2,4-difluorobenzylidene)-4-piperidone, a novel compound that affects pancreatic cancer growth and angiogenesis.Mol Cancer Ther. 2011 Nov;10(11):2146-56. doi: 10.1158/1535-7163.MCT-11-0399. Epub 2011 Sep 2. Mol Cancer Ther. 2011. PMID: 21890747 Free PMC article.
-
HES1-mediated inhibition of Notch1 signaling by a Gemini vitamin D analog leads to decreased CD44(+)/CD24(-/low) tumor-initiating subpopulation in basal-like breast cancer.J Steroid Biochem Mol Biol. 2015 Apr;148:111-21. doi: 10.1016/j.jsbmb.2014.12.013. Epub 2014 Dec 23. J Steroid Biochem Mol Biol. 2015. PMID: 25541438 Free PMC article.
-
Targeting notch to eradicate pancreatic cancer stem cells for cancer therapy.Anticancer Res. 2011 Apr;31(4):1105-13. Anticancer Res. 2011. PMID: 21508353 Review.
-
Notch Signaling in Breast Cancer: A Role in Drug Resistance.Cells. 2020 Sep 29;9(10):2204. doi: 10.3390/cells9102204. Cells. 2020. PMID: 33003540 Free PMC article. Review.
Cited by
-
Super-enhancers: novel target for pancreatic ductal adenocarcinoma.Oncotarget. 2019 Feb 22;10(16):1554-1571. doi: 10.18632/oncotarget.26704. eCollection 2019 Feb 22. Oncotarget. 2019. PMID: 30899425 Free PMC article.
-
Long-Term Inhibition of Notch in A-375 Melanoma Cells Enhances Tumor Growth Through the Enhancement of AXIN1, CSNK2A3, and CEBPA2 as Intermediate Genes in Wnt and Notch Pathways.Front Oncol. 2020 Jun 30;10:531. doi: 10.3389/fonc.2020.00531. eCollection 2020. Front Oncol. 2020. PMID: 32695658 Free PMC article.
-
CD133+CD24lo defines a 5-Fluorouracil-resistant colon cancer stem cell-like phenotype.Oncotarget. 2016 Nov 29;7(48):78698-78712. doi: 10.18632/oncotarget.12168. Oncotarget. 2016. PMID: 27659530 Free PMC article.
-
Therapeutic Status and Available Strategies in Pancreatic Ductal Adenocarcinoma.Biomedicines. 2021 Feb 11;9(2):178. doi: 10.3390/biomedicines9020178. Biomedicines. 2021. PMID: 33670230 Free PMC article. Review.
-
Repurposing Established Compounds to Target Pancreatic Cancer Stem Cells (CSCs).Med Sci (Basel). 2017 Jun 19;5(2):14. doi: 10.3390/medsci5020014. Med Sci (Basel). 2017. PMID: 29099030 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. - PubMed
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer research. 2014;74:2913–2921. - PubMed
-
- Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. Journal of clinical oncology. 1997;15:2403–2413. - PubMed
-
- Burris H, Storniolo AM. Assessing clinical benefit in the treatment of pancreas cancer: gemcitabine compared to 5-fluorouracil. Eur J Cancer. 1997;33:S18–22. - PubMed
-
- Foster BJ, Clagett-Carr K, Shoemaker DD, Suffness M, Plowman J, Trissel LA, Grieshaber CK, Leyland-Jones B. Echinomycin: the first bifunctional intercalating agent in clinical trials. Investigational new drugs. 1985;3:403–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

