Selective ORAI1 Inhibition Ameliorates Autoimmune Central Nervous System Inflammation by Suppressing Effector but Not Regulatory T Cell Function
- PMID: 26673135
- PMCID: PMC4707123
- DOI: 10.4049/jimmunol.1501406
Selective ORAI1 Inhibition Ameliorates Autoimmune Central Nervous System Inflammation by Suppressing Effector but Not Regulatory T Cell Function
Abstract
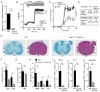
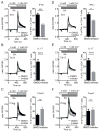
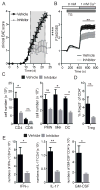
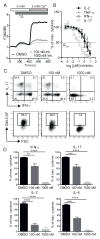
The function of CD4(+) T cells is dependent on Ca(2+) influx through Ca(2+) release-activated Ca(2+) (CRAC) channels formed by ORAI proteins. To investigate the role of ORAI1 in proinflammatory Th1 and Th17 cells and autoimmune diseases, we genetically and pharmacologically modulated ORAI1 function. Immunization of mice lacking Orai1 in T cells with MOG peptide resulted in attenuated severity of experimental autoimmune encephalomyelitis (EAE). The numbers of T cells and innate immune cells in the CNS of ORAI1-deficient animals were strongly reduced along with almost completely abolished production of IL-17A, IFN-γ, and GM-CSF despite only partially reduced Ca(2+) influx. In Th1 and Th17 cells differentiated in vitro, ORAI1 was required for cytokine production but not the expression of Th1- and Th17-specific transcription factors T-bet and RORγt. The differentiation and function of induced regulatory T cells, by contrast, was independent of ORAI1. Importantly, induced genetic deletion of Orai1 in adoptively transferred, MOG-specific T cells was able to halt EAE progression after disease onset. Likewise, treatment of wild-type mice with a selective CRAC channel inhibitor after EAE onset ameliorated disease. Genetic deletion of Orai1 and pharmacological ORAI1 inhibition reduced the leukocyte numbers in the CNS and attenuated Th1/Th17 cell-mediated cytokine production. In human CD4(+) T cells, CRAC channel inhibition reduced the expression of IL-17A, IFN-γ, and other cytokines in a dose-dependent manner. Taken together, these findings support the conclusion that Th1 and Th17 cell function is particularly dependent on CRAC channels, which could be exploited as a therapeutic approach to T cell-mediated autoimmune diseases.
Copyright © 2016 by The American Association of Immunologists, Inc.
Conflict of interest statement
Figures


References
-
- Ben-Nun A, Wekerle H, Cohen IR. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981;11:195–199. - PubMed
-
- Kuchroo VK, Anderson AC, Waldner H, Munder M, Bettelli E, Nicholson LB. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu Rev Immunol. 2002;20:101–123. - PubMed
-
- Traugott U, Lebon P. Interferon-gamma and Ia antigen are present on astrocytes in active chronic multiple sclerosis lesions. J Neurol Sci. 1988;84:257–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

