Reversible sarcopenia in patients with gastrointestinal stromal tumor treated with imatinib
- PMID: 26673372
- PMCID: PMC4670743
- DOI: 10.1002/jcsm.12047
Reversible sarcopenia in patients with gastrointestinal stromal tumor treated with imatinib
Abstract
Background: Imatinib is a long-term, oral, targeted therapy for high-risk resected and advanced gastrointestinal stromal tumours (GIST). It is known that sarcopenia affects prognosis and treatment tolerance in patients with various solid cancers. We analysed lumbar skeletal muscle index changes in imatinib-treated GIST patients. Imatinib tolerance was also assessed to evaluate the influence of pre-treatment sarcopenia.
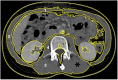
Methods: Thirty-one patients with advanced (n = 16) or high-risk resected (n = 15) GIST treated with imatinib (400 mg/day) were analysed retrospectively. Lumbar skeletal muscle indexes were evaluated on computed tomography images obtained before starting imatinib for all patients and at 6 months for those initially sarcopenic. Sarcopenia was defined using consensual cutoffs. Imatinib-induced toxicities were assessed after 3 months of administration.
Results: Twelve (38.7%) of the 31 patients were sarcopenic, including one unassessable at 6 months. Seven (63.6%) of the 11 assessable sarcopenic patients became non-sarcopenic after 6 months of imatinib. Pre-treatment sarcopenia was not associated with grades 3-4 toxicities, but the mean number of all-grade toxicities per sarcopenic patient was significantly higher for those non-sarcopenic (4.1 vs. 1.7, respectively, p < 0.01) after 3 months of treatment. Grades 1-2 anaemia and grades 1-2 fatigue were more frequent for sarcopenic than non-sarcopenic patients (83% vs. 26%, P < 0.01 and 42% vs. 5%, P = 0.02, respectively).
Conclusions: Sarcopenia is reversible in some GIST patients treated with imatinib. Pre-imatinib sarcopenia is predictive of non-severe toxicities, particularly anaemia and fatigue.
Keywords: Body composition; C-kit inhibitors; Gastrointestinal stromal tumours; Imatinib; Sarcopenia; Toxicity.
Figures


References
-
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. - PubMed
-
- ESMO / European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2012;23:vii49–vii55. - PubMed
-
- Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay J-Y, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–1134. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

