Phase II/III weekly nab-paclitaxel plus gemcitabine or carboplatin versus gemcitabine/carboplatin as first-line treatment of patients with metastatic triple-negative breast cancer (the tnAcity study): study protocol for a randomized controlled trial
- PMID: 26673577
- PMCID: PMC4682258
- DOI: 10.1186/s13063-015-1101-7
Phase II/III weekly nab-paclitaxel plus gemcitabine or carboplatin versus gemcitabine/carboplatin as first-line treatment of patients with metastatic triple-negative breast cancer (the tnAcity study): study protocol for a randomized controlled trial
Erratum in
-
Erratum to: 'Phase II/III weekly nab-paclitaxel plus gemcitabine or carboplatin versus gemcitabine/carboplatin as first-line treatment of patients with metastatic triple-negative breast cancer (the tnAcity study): study protocol for a randomized controlled trial.Trials. 2016 Feb 3;17:63. doi: 10.1186/s13063-016-1195-6. Trials. 2016. PMID: 26841937 Free PMC article. No abstract available.
Abstract
Background: Triple-negative breast cancer is an aggressive disease with unmet clinical needs. In a phase III study of patients with metastatic triple-negative breast cancer, first-line gemcitabine/carboplatin resulted in a median progression-free survival of 4.6 months. nab-paclitaxel-based regimens (with gemcitabine or carboplatin±bevacizumab) also demonstrated efficacy and safety in first-line phase II trials of human epidermal growth factor receptor 2-negative metastatic breast cancer.
Trial design: In this international, multicenter, open-label, randomized phase II/III trial, the efficacy and safety of first-line nab-paclitaxel with gemcitabine or with carboplatin will be compared with gemcitabine/carboplatin (control arm) for metastatic triple-negative breast cancer.
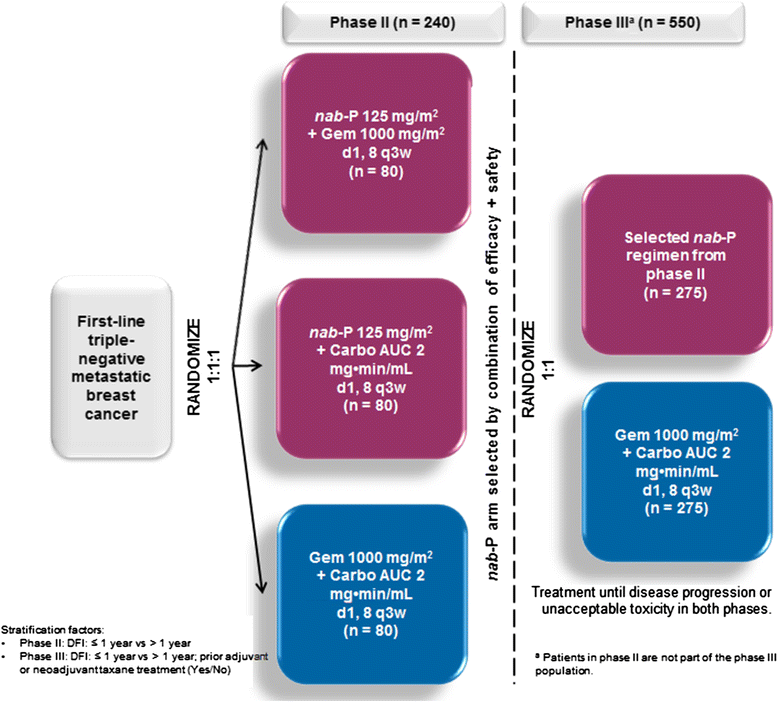
Methods: In the phase II portion, 240 patients with measurable metastatic triple-negative breast cancer and treatment-naive for metastatic disease will be randomized 1:1:1 (stratified by disease-free interval: ≤1 versus>1 year) to nab-paclitaxel 125 mg/m2 plus gemcitabine 1000 mg/m2, nab-paclitaxel 125 mg/m2 plus carboplatin area under the curve 2 mg×min/mL, or gemcitabine 1000 mg/m2 plus carboplatin area under the curve 2 mg×min/mL, all given on days 1 and 8 of a 21-day cycle. Investigator-assessed progression-free survival (primary endpoint), overall response rate, overall survival, and safety will be assessed. A ranking algorithm of five efficacy and safety parameters will be used to pick the "winner" of the nab-paclitaxel regimens. In the phase III portion, 550 patients will be randomized 1:1 (stratified by disease-free interval: ≤1 versus >1 year, and prior adjuvant/neoadjuvant taxane use) to the nab-paclitaxel combination arm selected from the phase II portion or to the control arm. Patients in phase II will not be part of the phase III population. The phase III primary endpoint is blinded, independently-assessed progression-free survival; secondary endpoints include blinded, independently-assessed overall response rate, overall survival, disease control rate, duration of response, and safety. Biomarker and circulating tumor-cell exploratory analyses and quality-of-life assessments will also be performed. A list of approving ethical bodies was provided in Additional file 1.
Discussion: The tnAcity trial aims to identify a new standard cytotoxic chemotherapy regimen for first-line treatment of metastatic triple-negative breast cancer.
Trial registration: ClinicalTrials.gov: NCT01881230 . Date of registration: 17 June 2013.
Figures
Similar articles
-
nab-Paclitaxel plus carboplatin or gemcitabine versus gemcitabine plus carboplatin as first-line treatment of patients with triple-negative metastatic breast cancer: results from the tnAcity trial.Ann Oncol. 2018 Aug 1;29(8):1763-1770. doi: 10.1093/annonc/mdy201. Ann Oncol. 2018. PMID: 29878040 Free PMC article. Clinical Trial.
-
Comparison of Neoadjuvant Nab-Paclitaxel+Carboplatin vs Nab-Paclitaxel+Gemcitabine in Triple-Negative Breast Cancer: Randomized WSG-ADAPT-TN Trial Results.J Natl Cancer Inst. 2018 Jun 1;110(6):628-637. doi: 10.1093/jnci/djx258. J Natl Cancer Inst. 2018. PMID: 29228315 Clinical Trial.
-
Trilaciclib plus chemotherapy versus chemotherapy alone in patients with metastatic triple-negative breast cancer: a multicentre, randomised, open-label, phase 2 trial.Lancet Oncol. 2019 Nov;20(11):1587-1601. doi: 10.1016/S1470-2045(19)30616-3. Epub 2019 Sep 28. Lancet Oncol. 2019. PMID: 31575503 Clinical Trial.
-
Nab-paclitaxel for the treatment of triple-negative breast cancer: Rationale, clinical data and future perspectives.Cancer Treat Rev. 2016 Nov;50:129-141. doi: 10.1016/j.ctrv.2016.09.004. Epub 2016 Sep 12. Cancer Treat Rev. 2016. PMID: 27665540 Review.
-
Gemcitabine and paclitaxel in metastatic breast cancer: a review.Oncology (Williston Park). 2004 Dec;18(14 Suppl 12):8-12. Oncology (Williston Park). 2004. PMID: 15685819 Review.
Cited by
-
Proteomes from AMPK-inhibited peripheral blood mononuclear cells suppress the progression of breast cancer and bone metastasis.Theranostics. 2023 Feb 5;13(4):1247-1263. doi: 10.7150/thno.80294. eCollection 2023. Theranostics. 2023. PMID: 36923539 Free PMC article.
-
Erratum to: 'Phase II/III weekly nab-paclitaxel plus gemcitabine or carboplatin versus gemcitabine/carboplatin as first-line treatment of patients with metastatic triple-negative breast cancer (the tnAcity study): study protocol for a randomized controlled trial.Trials. 2016 Feb 3;17:63. doi: 10.1186/s13063-016-1195-6. Trials. 2016. PMID: 26841937 Free PMC article. No abstract available.
-
Weekly albumin-bound paclitaxel/cisplatin versus gemcitabine/cisplatin as first-line therapy for patients with advanced non-small-cell lung cancer: A phase II open-label clinical study.Chin J Cancer Res. 2019 Apr;31(2):339-348. doi: 10.21147/j.issn.1000-9604.2019.02.08. Chin J Cancer Res. 2019. PMID: 31156304 Free PMC article.
-
Recent Advancements and Strategies for Overcoming the Blood-Brain Barrier Using Albumin-Based Drug Delivery Systems to Treat Brain Cancer, with a Focus on Glioblastoma.Polymers (Basel). 2023 Oct 2;15(19):3969. doi: 10.3390/polym15193969. Polymers (Basel). 2023. PMID: 37836018 Free PMC article. Review.
-
nab-Paclitaxel plus carboplatin or gemcitabine versus gemcitabine plus carboplatin as first-line treatment of patients with triple-negative metastatic breast cancer: results from the tnAcity trial.Ann Oncol. 2018 Aug 1;29(8):1763-1770. doi: 10.1093/annonc/mdy201. Ann Oncol. 2018. PMID: 29878040 Free PMC article. Clinical Trial.
References
-
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109(9):1721–8. doi: 10.1002/cncr.22618. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials